DNA binding properties of the adenovirus DNA replication priming protein pTP
- PMID: 12799455
- PMCID: PMC162239
- DOI: 10.1093/nar/gkg405
DNA binding properties of the adenovirus DNA replication priming protein pTP
Abstract
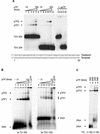
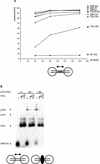
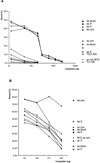
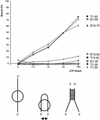
The precursor terminal protein pTP is the primer for the initiation of adenovirus (Ad) DNA replication and forms a heterodimer with Ad DNA polymerase (pol). Pol can couple dCTP to pTP directed by the fourth nucleotide of the viral genome template strand in the absence of other replication proteins, which suggests that pTP/pol binding destabilizes the origin or stabilizes an unwound state. We analyzed the contribution of pTP to pTP/pol origin binding using various DNA oligonucleotides. We show that two pTP molecules bind cooperatively to short DNA duplexes, while longer DNA fragments are bound by single pTP molecules as well. Cooperative binding to short duplexes is DNA sequence independent and most likely mediated by protein/protein contacts. Furthermore, we observed that pTP binds single-stranded (ss)DNA with a minimal length of approximately 35 nt and that random ssDNA competed 25-fold more efficiently than random duplex DNA for origin binding by pTP. Remarkably, short DNA fragments with two opposing single strands supported monomeric pTP binding. pTP did not stimulate, but inhibited strand displacement by the Ad DNA binding and unwinding protein DBP. These observations suggest a mechanism in which the ssDNA affinity of pTP stabilizes Ad pol on partially unwound origin DNA.
Figures





Similar articles
-
The adenovirus priming protein pTP contributes to the kinetics of initiation of DNA replication.Nucleic Acids Res. 2004 Jul 25;32(13):3913-20. doi: 10.1093/nar/gkh726. Print 2004. Nucleic Acids Res. 2004. PMID: 15273278 Free PMC article.
-
Molecular architecture of adenovirus DNA polymerase and location of the protein primer.J Virol. 2002 Aug;76(16):8200-7. doi: 10.1128/jvi.76.16.8200-8207.2002. J Virol. 2002. PMID: 12134025 Free PMC article.
-
Properties of the adenovirus DNA polymerase.J Biol Chem. 1984 Aug 10;259(15):9487-95. J Biol Chem. 1984. PMID: 6540263
-
Adenovirus DNA replication: protein priming, jumping back and the role of the DNA binding protein DBP.Curr Top Microbiol Immunol. 2003;272:187-211. doi: 10.1007/978-3-662-05597-7_7. Curr Top Microbiol Immunol. 2003. PMID: 12747551 Review.
-
Protein-nucleic acid interactions in bacteriophage phi 29 DNA replication.FEMS Microbiol Rev. 1995 Aug;17(1-2):73-82. doi: 10.1111/j.1574-6976.1995.tb00189.x. FEMS Microbiol Rev. 1995. PMID: 7669351 Review.
Cited by
-
Dissecting the role of the ϕ29 terminal protein DNA binding residues in viral DNA replication.Nucleic Acids Res. 2015 Mar 11;43(5):2790-801. doi: 10.1093/nar/gkv127. Epub 2015 Feb 26. Nucleic Acids Res. 2015. PMID: 25722367 Free PMC article.
-
Relationship between adenovirus DNA replication proteins and nucleolar proteins B23.1 and B23.2.J Gen Virol. 2007 Dec;88(Pt 12):3244-3248. doi: 10.1099/vir.0.83196-0. J Gen Virol. 2007. PMID: 18024892 Free PMC article.
-
The adenovirus priming protein pTP contributes to the kinetics of initiation of DNA replication.Nucleic Acids Res. 2004 Jul 25;32(13):3913-20. doi: 10.1093/nar/gkh726. Print 2004. Nucleic Acids Res. 2004. PMID: 15273278 Free PMC article.
-
Human adenovirus type 5 vectors deleted of early region 1 (E1) undergo limited expression of early replicative E2 proteins and DNA replication in non-permissive cells.PLoS One. 2017 Jul 10;12(7):e0181012. doi: 10.1371/journal.pone.0181012. eCollection 2017. PLoS One. 2017. PMID: 28700677 Free PMC article.
-
Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid Suppresses Human Adenovirus Gene Expression and Replication.J Virol. 2019 May 29;93(12):e00088-19. doi: 10.1128/JVI.00088-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944181 Free PMC article.
References
-
- de Jong R.N. and van der Vliet,P.C. (1999) Mechanism of DNA replication in eukaryotic cells: cellular host factors stimulating adenovirus DNA replication. Gene, 236, 1–12. - PubMed
-
- Hay R.T., Freeman,A., Leith,I., Monaghan,A. and Webster,A. (1995) Molecular interactions during adenovirus DNA replication. Curr. Top. Microbiol. Immunol., 199, 31–48. - PubMed
-
- Dekker J., Kanellopoulos,P.N., Loonstra,A.K., van Oosterhout,J.A., Leonard,K., Tucker,P.A. and van der Vliet,P.C. (1997) Multimerization of the adenovirus DNA-binding protein is the driving force for ATP-independent DNA unwinding during strand displacement synthesis. EMBO J., 16, 1455–1463. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

