Conditional human VEGF-mediated vascularization in chicken embryos using a novel temperature-inducible gene regulation (TIGR) system
- PMID: 12799458
- PMCID: PMC162344
- DOI: 10.1093/nar/gng069
Conditional human VEGF-mediated vascularization in chicken embryos using a novel temperature-inducible gene regulation (TIGR) system
Abstract
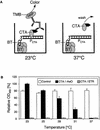
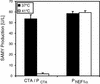
Advanced heterologous transcription control systems for adjusting desired transgene expression are essential for gene function assignments, drug discovery, manufacturing of difficult to produce protein pharmaceuticals and precise dosing of gene-based therapeutic interventions. Conversion of the Streptomyces albus heat shock response regulator (RheA) into an artificial eukaryotic transcription factor resulted in a vertebrate thermosensor (CTA; cold-inducible transactivator), which is able to adjust transcription initiation from chimeric target promoters (P(CTA)) in a low-temperature- inducible manner. Evaluation of the temperature-dependent CTA-P(CTA) interaction using a tailored ELISA-like cell-free assay correlated increased affinity of CTA for P(CTA) with temperature downshift. The temperature-inducible gene regulation (TIGR) system enabled tight repression in the chicken bursal B-cell line DT40 at 41 degrees C as well as precise titration of model product proteins up to maximum expression at or below 37 degrees C. Implantation of microencapsulated DT40 cells engineered for TIGR-controlled expression of the human vascular endothelial growth factor A (hVEGF121) provided low-temperature-induced VEGF-mediated vascularization in chicken embryos.
Figures



References
-
- Iba K. (2002) Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol., 53, 225–245. - PubMed
-
- Fujita J. (1999) Cold shock response in mammalian cells. J. Mol. Microbiol. Biotechnol., 1, 243–255. - PubMed
-
- Yamanaka K. (1999) Cold shock response in Escherichia coli. J. Mol. Microbiol. Biotechnol., 1, 193–202. - PubMed
-
- Hurme R. and Rhen,M. (1998) Temperature sensing in bacterial gene regulation—what it all boils down to. Mol. Microbiol., 30, 1–6. - PubMed
-
- Johansson J., Mandin,P., Renzoni,A., Chiaruttini,C., Springer,M. and Cossart,P. (2002) An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell, 110, 551–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

