A dose- and schedule-finding study of darbepoetin alpha for the treatment of chronic anaemia of cancer
- PMID: 12799626
- PMCID: PMC2741110
- DOI: 10.1038/sj.bjc.6600994
A dose- and schedule-finding study of darbepoetin alpha for the treatment of chronic anaemia of cancer
Abstract
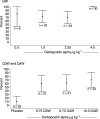
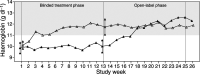
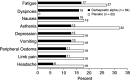
A multicentre study evaluated the efficacy and safety of darbepoetin alpha administered weekly (QW), every 3 weeks (Q3W), and every 4 weeks (Q4W) to anaemic patients with cancer not concurrently receiving chemotherapy or radiotherapy. The QW portion (n=102) was an open-label, sequential, dose-escalation design; cohorts received darbepoetin alpha QW by subcutaneous (s.c.) injection at 0.5, 1.0, 2.25, or 4.5 micro g kg(-1) week(-1) for 12 weeks. The 12-week placebo-controlled, double-blind Q3W (6.75 micro g kg(-1)) and Q4W (6.75 or 10.0 micro g kg(-1)) schedules (n=86), which enrolled different patients, took place after the QW schedule and were followed by a 12-week, open-label phase. Patients were evaluated for change in haemoglobin end points and red blood cell transfusions, serum darbepoetin alpha concentration, and safety. Selected domains of health-related quality of life (HRQOL) were measured. With QW dosing, at least 70% of each cohort had a haemoglobin increase from baseline of > or =2 g dl(-1) or a concentration > or =12 g dl(-1) (haematopoietic response). In the 4.5 micro g kg(-1) QW cohort, all patients achieved a haematopoietic response (100%; 95% confidence interval (CI)=100, 100). In the Q3W and Q4W schedules, all cohorts had at least 60% of patients who achieved a haematopoietic response. Darbepoetin alpha effectively increases haemoglobin concentration when given QW, Q3W, or Q4W. Less-frequent administration may benefit patients with chronic anaemia of cancer and their caregivers alike.
Figures
References
-
- Abels RI (1992) Use of recombinant human erythropoietin in the treatment of anemia in patients who have cancer. Semin Oncol 19: 29–35 - PubMed
-
- Abels RI, Larholt KM, Krantz KD, Bryant EC (1991) Recombinant human erythropoietin (rHuEPO) for the treatment of the anemia of cancer, In Blood Cell Growth Factors: Their Present and Future Use in Hematology and Oncology. Proceedings of the Beijing Symposium., Murphy Jr MJ (ed.), pp 121–142. Dayton, OH, USA: alphaMed Press
-
- Cartwright GE (1966) The anemia of chronic disorders. Semin Hematol 3: 351–375 - PubMed
-
- Cella D (1997a) The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol 34: 13–19 - PubMed
-
- Cella D (1997b) Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Scales, Version 4. Evanston, IL Center on Outcomes, Research and Education, Evanston Northwestern Healthcare and Northwestern University
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

