Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro
- PMID: 12799645
- PMCID: PMC2741108
- DOI: 10.1038/sj.bjc.6600986
Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro
Abstract
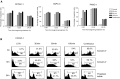
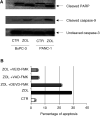
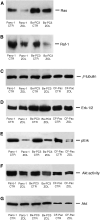
Bisphosphonates (BPs) are an emerging class of drugs mostly used in the palliative care of cancer patients. We investigated the in vitro activity of the most potent antiresorptive BP, zoledronic acid (ZOL), on the growth and survival of three human pancreatic cancer (PC) cell lines (BxPC-3, CFPAC-1 and PANC-1). Pancreatic cancer frequently has a dysregulated p21(ras) pathway and therefore appears to be a suitable target for BPs that interfere with the prenylation of small GTP-binding proteins such as p21(ras). We found that ZOL induces growth inhibition (IC(50):10-50 micro M) and apoptotic death of PC cells. The proapoptotic effect was correlated to cleavage/activation of caspase-9 and poly(ADP)-ribose polymerase, but not of caspase-3. Moreover, we studied the p21(ras) signalling in cells exposed to ZOL and detected a reduction of p21(ras) and Raf-1 content and functional downregulation of the terminal enzyme ERK/MAPkinase and of the pKB/Akt survival pathway. Finally, we observed that ZOL induces significant cytoskeletal rearrangements. In conclusion, we demonstrated that ZOL induces growth inhibition and apoptosis on PC cells and interferes with growth and survival pathways downstream to p21(ras). These findings might be relevant for expanding application of BPs in cancer treatment.
Figures


Similar articles
-
The farnesyl transferase inhibitor R115777 (Zarnestra) synergistically enhances growth inhibition and apoptosis induced on epidermoid cancer cells by Zoledronic acid (Zometa) and Pamidronate.Oncogene. 2004 Sep 9;23(41):6900-13. doi: 10.1038/sj.onc.1207814. Oncogene. 2004. PMID: 15286715
-
Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells.Cancer Res. 2004 Aug 1;64(15):5291-300. doi: 10.1158/0008-5472.CAN-04-1112. Cancer Res. 2004. PMID: 15289335
-
Zoledronic acid produces antitumor effects on mesothelioma through apoptosis and S-phase arrest in p53-independent and Ras prenylation-independent manners.J Thorac Oncol. 2012 May;7(5):873-82. doi: 10.1097/JTO.0b013e31824c7d43. J Thorac Oncol. 2012. PMID: 22481236
-
The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway.Mol Cell Biol. 2003 Jan;23(2):543-54. doi: 10.1128/MCB.23.2.543-554.2003. Mol Cell Biol. 2003. PMID: 12509453 Free PMC article.
-
Zoledronic acid - a multiplicity of anti-cancer action.Curr Med Chem. 2007;14(20):2126-35. doi: 10.2174/092986707781389600. Curr Med Chem. 2007. PMID: 17691952 Review.
Cited by
-
Zoledronic acid in metastatic osteosarcoma: encouraging progression free survival in four consecutive patients.Clin Sarcoma Res. 2016 Apr 28;6:6. doi: 10.1186/s13569-016-0046-2. eCollection 2016. Clin Sarcoma Res. 2016. PMID: 27127605 Free PMC article.
-
Nanoparticles for the delivery of zoledronic acid to prostate cancer cells: a comparative analysis through time lapse video-microscopy technique.Cancer Biol Ther. 2014;15(11):1524-32. doi: 10.4161/15384047.2014.955989. Cancer Biol Ther. 2014. PMID: 25482949 Free PMC article.
-
Preclinical evaluation of an innovative anti-TAM approach based on zoledronate-loaded erythrocytes.Drug Deliv Transl Res. 2018 Oct;8(5):1355-1364. doi: 10.1007/s13346-018-0560-2. Drug Deliv Transl Res. 2018. PMID: 30014237
-
Acute tumor lysis syndrome triggered by zoledronic Acid in a patient with metastatic lung adenocarcinoma.Med Oncol. 2005;22(2):203-6. doi: 10.1385/MO:22:2:203. Med Oncol. 2005. PMID: 15965285
-
Low-dose zoledronate for the treatment of bone metastasis secondary to prostate cancer.Cancer Cell Int. 2019 Feb 8;19:28. doi: 10.1186/s12935-019-0745-x. eCollection 2019. Cancer Cell Int. 2019. PMID: 30787671 Free PMC article.
References
-
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M (1988) Most human carcinomas of the exocrine pancreas contain mutant c-Kras gene. Cell 53: 549–554 - PubMed
-
- Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A (1998) In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 12: 220–229 - PubMed
-
- Benford HL, Frith JC, Auriola S, Monkkonen J, Rogers MJ (1999) Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol 56: 131–140 - PubMed
-
- Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ (2001) Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone 28: 465–473 - PubMed
-
- Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, Lipton A, Keller A, Ballester O, Kovacs M, Blacklock H, Bell R, Simeone JF, Reitsma DJ, Heffernan M, Seaman J, Knight RD (1998) Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 16: 593–602 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

