Loss of function and impaired degradation of a cataract-associated mutant connexin50
- PMID: 12800976
- PMCID: PMC2763359
- DOI: 10.1078/0171-9335-00316
Loss of function and impaired degradation of a cataract-associated mutant connexin50
Abstract
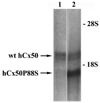
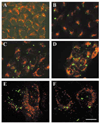
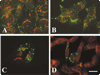
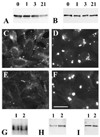
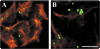
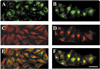
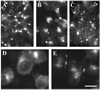
A mutant human connexin50 (hCx50), hCx50P88S, has been linked to cataracts inherited as an autosomal dominant trait. The functional, biochemical and cellular behavior of wild-type and mutant hCx50 were examined in transfected cells. hCx50P88S was unable to induce gap junctional currents by itself, and it abolished gap junctional currents when co-expressed with wild-type (wt) hCx50. Cells transfected with hCx50P88S showed cytoplasmic accumulations of Cx50 immunoreactivity in addition to staining at appositional membranes; these accumulations did not significantly co-localize with markers for the endoplasmic reticulum, Golgi apparatus, lysosomes, endosomes or vimentin filaments. Immunoelectron microscopy studies localized hCx50P88S to cytoplasmic membrane stacks in close vicinity to the endoplasmic reticulum. In contrast, aggresome-like accumulations were induced by treatment of wt hCx50-transfected cells with proteasomal inhibitors. The formation of hCx50P88S accumulations in transiently transfected cells was not blocked by treatment with Brefeldin A suggesting that they form before Cx50 transits through the Golgi apparatus to the plasma membrane. Treatment of HeLa-hCx50P88S cells with cycloheximide demonstrated the presence of a very stable pool of hCx50P88S. Taken together, these results suggest that the P to S mutation at amino acid residue 88 causes a defect that leads to decreased degradation and subsequent accumulation of hCx50P88S in a cellular structure different from aggresomes.
Figures




References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. - PubMed
-
- Church RL, Wang JH, Steele E. The human lens intrinsic membrane protein MP70 (Cx50) gene – clonal analysis and chromosome mapping. Curr. Eye Res. 1995;14:215–221. - PubMed
-
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature sensitive. Nature. 1992;358:761–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

