Pathogenesis of axonal dystrophy and demyelination in alphaA-crystallin-expressing transgenic mice
- PMID: 12801283
- PMCID: PMC2517547
- DOI: 10.1046/j.1365-2613.2003.00340.x
Pathogenesis of axonal dystrophy and demyelination in alphaA-crystallin-expressing transgenic mice
Abstract
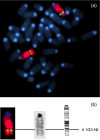
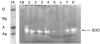
We recently described a transgenic mouse strain overexpressing hamster alphaA-crystallin, a small heat shock protein, under direction of the hamster vimentin promoter. As a result myelin was degraded and axonal dystrophy in both central nervous system (especially spinal cord) and peripheral nervous system occurred. Homozygous transgenic mice developed hind limb paralysis after 8 weeks of age and displayed progressive loss of myelin and axonal dystrophy in both the central and peripheral nervous system with ongoing age. Pathologically the phenotype resembled, to a certain extent, neuroaxonal dystrophy. The biochemical findings presented in this paper (activity of the enzymes superoxide dismutase, catalase and transglutamase, myelin protein zero expression levels and blood sugar levels) confirm this pathology and exclude other putative pathologies like Amyothrophic Lateral Sclerosis and Hereditary Motor and Sensory Neuropathy. Consequently, an excessive cytoplasmic accumulation of the transgenic protein or a disturbance of the normal metabolism are considered to cause the observed neuropathology. Therefore, extra-ocular alphaA-crystallin-expressing transgenic mice may serve as a useful animal model to study neuroaxonal dystrophy.
Figures


References
-
- Aizawa H, Kimura T, Hashimoto K, Yahara O, Okamoto K, Kikuchi K. Basophilic cytoplasmic inclusions in a case of sporadic juvenile amyotrophiclateral sclerosis. J. Neurol. Sci. 2000;176:109–113. - PubMed
-
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. - PubMed
-
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. - PubMed
-
- Bhat SP, Nagineni CN. αB Subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biophys. Res. Commun. 1989;158:319–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

