Risk of valvular heart disease associated with use of fenfluramine
- PMID: 12801402
- PMCID: PMC194859
- DOI: 10.1186/1471-2261-3-5
Risk of valvular heart disease associated with use of fenfluramine
Abstract
Background: Estimates of excess risk of valvular heart disease among prior users of fenfluramine and dexfenfluramine have varied widely. Two major forms of bias appear to contribute to this variability and also result in a systematic under-estimation of risk. The first, a form of nondifferential misclassification, is the result of including background, prevalent cases among both exposed and unexposed persons in calculations of risk. The second bias results from not considering the relatively short duration of exposure to drugs.
Methods: We examined data from all available echocardiographic studies reporting the prevalence of aortic regurgitation (AR) and mitral regurgitation (MR) among persons exposed to fenfluramine or dexfenfluramine and a suitable control group. We also included one study in which previously existing AR or MR had been excluded. We corrected for background prevalent cases, estimated incidence rates in unexposed persons, and performed a person-years analysis of apparent incidence rates based on exposure time to provide an unbiased estimate of relative risk.
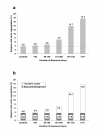
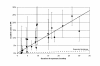
Results: Appearance of new AR was strongly related to duration of exposure (R2 = 0.75, p < 0.0001). The summary relative risk for mild or greater AR was 19.6 (95% CI 16.3-23.5, p < 0.00001); for moderate or greater MR it was 5.9 (95% CI 4.0-8.6, p < 0.00001).
Conclusion: These findings provide strong support for the view that fenfluramine and dexfenfluramine are potent causal factors in the development of both aortic and mitral valvular heart disease.
Figures


Similar articles
-
Valvular abnormalities and cardiovascular status following exposure to dexfenfluramine or phentermine/fenfluramine.JAMA. 2000 Apr 5;283(13):1703-9. doi: 10.1001/jama.283.13.1703. JAMA. 2000. PMID: 10755496
-
Echocardiographic prevalence of mitral and/or aortic regurgitation in patients exposed to either fenfluramine-phentermine combination or to dexfenfluramine.Am J Cardiol. 1999 Dec 1;84(11):1335-8. doi: 10.1016/s0002-9149(99)00567-6. Am J Cardiol. 1999. PMID: 10614800 Clinical Trial.
-
Serial echocardiographic and clinical evaluation of valvular regurgitation before, during, and after treatment with fenfluramine or dexfenfluramine and mazindol or phentermine.Obes Res. 1999 Jul;7(4):313-22. doi: 10.1002/j.1550-8528.1999.tb00414.x. Obes Res. 1999. PMID: 10440587
-
Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies.Am Heart J. 2002 Dec;144(6):1065-73. doi: 10.1067/mhj.2002.126733. Am Heart J. 2002. PMID: 12486432 Review.
-
[About the settlement of litigations concerning the harm caused by benfluorex (Mediator®)].Presse Med. 2013 Apr;42(4 Pt 1):411-8. doi: 10.1016/j.lpm.2013.02.310. Epub 2013 Mar 11. Presse Med. 2013. PMID: 23490638 Review. French.
Cited by
-
Binding and functional structure-activity similarities of 4-substituted 2,5-dimethoxyphenyl isopropylamine analogues at 5-HT2A and 5-HT2B serotonin receptors.Front Pharmacol. 2023 Jan 24;14:1101290. doi: 10.3389/fphar.2023.1101290. eCollection 2023. Front Pharmacol. 2023. PMID: 36762110 Free PMC article.
-
Reintroducing Fenfluramine as a Treatment for Seizures: Current Knowledge, Recommendations and Gaps in Understanding.Neuropsychiatr Dis Treat. 2023 Sep 26;19:2013-2025. doi: 10.2147/NDT.S417676. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 37790801 Free PMC article. Review.
-
Fenfluramine for Treatment-Resistant Seizures in Patients With Dravet Syndrome Receiving Stiripentol-Inclusive Regimens: A Randomized Clinical Trial.JAMA Neurol. 2020 Mar 1;77(3):300-308. doi: 10.1001/jamaneurol.2019.4113. JAMA Neurol. 2020. PMID: 31790543 Free PMC article. Clinical Trial.
-
Fenfluramine: A Review of Pharmacology, Clinical Efficacy, and Safety in Epilepsy.Children (Basel). 2022 Aug 2;9(8):1159. doi: 10.3390/children9081159. Children (Basel). 2022. PMID: 36010049 Free PMC article. Review.
-
Cardiovascular Effects of Antiseizure Medications for Epilepsy.CNS Drugs. 2025 Apr;39(4):383-401. doi: 10.1007/s40263-025-01163-x. Epub 2025 Feb 14. CNS Drugs. 2025. PMID: 39951223 Free PMC article. Review.
References
-
- Bowen R, Glicklich A, Khan M, Rasmussen S, Wadden T, Bilstad J, Graham D, Green L, Lumpkin M, O’Neill R, Sobel S, Hubbard VS, Yanovski S, Sopko G. Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services Interim Public Health Recommendations. MMWR. 1997;46:1061–1066. - PubMed
-
- Wadden TA, Berkowitz RI, Silvestry F, Vogt RA, St John Sutton MG, Stunkard AJ, Foster GD, Aber JL. The fen-phen finale: a study of weight loss and valvular heart disease. Obes Res. 1998;6:278–284. - PubMed
-
- Teramae CY, Connolly HM, Grogan M, Miller F. A., Jr. Diet drug-related cardiac valve disease: the Mayo Clinic echocardiographic laboratory experience. Mayo Clin Proc. 2000;75:456–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

