Matrix metalloproteinases: old dogs with new tricks
- PMID: 12801404
- PMCID: PMC193609
- DOI: 10.1186/gb-2003-4-6-216
Matrix metalloproteinases: old dogs with new tricks
Abstract
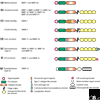
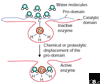
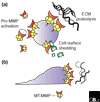
The matrix metalloproteinase family in humans comprises 23 enzymes, which are involved in many biological processes and diseases. It was previously thought that these enzymes acted only to degrade components of the extracellular matrix, but this view has changed with the discovery that non-extracellular-matrix molecules are also substrates.
Figures

References
-
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. - PubMed
-
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. - PubMed
-
- Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. - PubMed
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

