ScanImage: flexible software for operating laser scanning microscopes
- PMID: 12801419
- PMCID: PMC161784
- DOI: 10.1186/1475-925X-2-13
ScanImage: flexible software for operating laser scanning microscopes
Abstract
Background: Laser scanning microscopy is a powerful tool for analyzing the structure and function of biological specimens. Although numerous commercial laser scanning microscopes exist, some of the more interesting and challenging applications demand custom design. A major impediment to custom design is the difficulty of building custom data acquisition hardware and writing the complex software required to run the laser scanning microscope.
Results: We describe a simple, software-based approach to operating a laser scanning microscope without the need for custom data acquisition hardware. Data acquisition and control of laser scanning are achieved through standard data acquisition boards. The entire burden of signal integration and image processing is placed on the CPU of the computer. We quantitate the effectiveness of our data acquisition and signal conditioning algorithm under a variety of conditions. We implement our approach in an open source software package (ScanImage) and describe its functionality.
Conclusions: We present ScanImage, software to run a flexible laser scanning microscope that allows easy custom design.
Figures
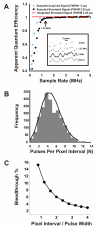
 ) of the signal (see inset) determined by digital integration. The black trace shows
) of the signal (see inset) determined by digital integration. The black trace shows  sampled at 200 kHz to 5 MHz.
sampled at 200 kHz to 5 MHz.  approaches 1 as the sample rate approaches 1 MHz. The blue points are the normalized apparent quantum efficiencies from an actual PMT illuminated by a constant light source (data pre-filtered at 330 kHz). ScanImage uses a sample rate of 1.25 MHz (arrow), which yields nearly ideal signal-to-noise
approaches 1 as the sample rate approaches 1 MHz. The blue points are the normalized apparent quantum efficiencies from an actual PMT illuminated by a constant light source (data pre-filtered at 330 kHz). ScanImage uses a sample rate of 1.25 MHz (arrow), which yields nearly ideal signal-to-noise  ≈ 1. This analysis is only valid for pulses that are al least 2.35 μs in duration. Inset: 20-μs pulse interval comprised of 2.35-μs events sampled at different rates. B) Histogram of the events in the pixel interval (bars) and Poisson fit (line). C) Model of bleedthrough of photons from adjacent pixels. A single-photon-pulse with a 2.5 μs FWHM was placed randomly in pixel intervals of widths 2 to 10 μs. The number of trials at each pixel interval was 10,000. The percent bleedthrough equaled 100*(1 - IPP/IEP) where IEP is the integral of the entire pulse and IPP is the integral of the region of the pulse that fell in the pixel interval. Shown is the mean value of the percent bleedthrough for each pixel time normalized with respect to the pulse width (Pixel Interval/Pulse Width). The error bars (SEM) were smaller than the marker points and were omitted.
≈ 1. This analysis is only valid for pulses that are al least 2.35 μs in duration. Inset: 20-μs pulse interval comprised of 2.35-μs events sampled at different rates. B) Histogram of the events in the pixel interval (bars) and Poisson fit (line). C) Model of bleedthrough of photons from adjacent pixels. A single-photon-pulse with a 2.5 μs FWHM was placed randomly in pixel intervals of widths 2 to 10 μs. The number of trials at each pixel interval was 10,000. The percent bleedthrough equaled 100*(1 - IPP/IEP) where IEP is the integral of the entire pulse and IPP is the integral of the region of the pulse that fell in the pixel interval. Shown is the mean value of the percent bleedthrough for each pixel time normalized with respect to the pulse width (Pixel Interval/Pulse Width). The error bars (SEM) were smaller than the marker points and were omitted.
References
-
- White EL, Keller A. Intrinsic circuitry involving the local axon collaterals of corticothalamic projection cells in mouse Sm1 cortex. J Comp Neurol. 1987;1987:13–26. - PubMed
-
- Goldstein SR, Hubin T, Smith TG. An improved, no moving parts video rate confocal microscope. Micron and Microscopica Acta. 1992;23:437–442. doi: 10.1016/0739-6260(92)90019-A. - DOI
-
- Pawley JB, ed . Plenum Press: New York. 3 1995. Handbook of Biological Confocal Microscopy.
-
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning microscopy. Science. 1990;248:73–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

