Antagonism of ghrelin receptor reduces food intake and body weight gain in mice
- PMID: 12801949
- PMCID: PMC1773718
- DOI: 10.1136/gut.52.7.947
Antagonism of ghrelin receptor reduces food intake and body weight gain in mice
Abstract
Background and aims: Ghrelin, an endogenous ligand for growth hormone secretagogue receptor (GHS-R), is an appetite stimulatory signal from the stomach with structural resemblance to motilin. We examined the effects of the gastric peptide ghrelin and GHS-R antagonists on energy balance and glycaemic control in mice.
Materials and methods: Body weight, fat mass, glucose, insulin, and gene expression of leptin, adiponectin, and resistin in white adipose tissue (WAT) were measured after repeated administrations of ghrelin under a high fat diet. Gastric ghrelin gene expression was assessed by northern blot analysis. Energy intake and gastric emptying were measured after administration of GHS-R antagonists. Repeated administration of GHS-R antagonist was continued for six days in ob/ob obese mice.
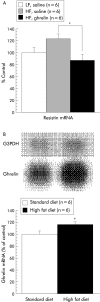
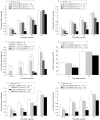
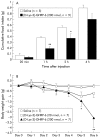
Results: Ghrelin induced remarkable adiposity and worsened glycaemic control under a high fat diet. Pair feeding inhibited this effect. Ghrelin elevated leptin mRNA expression and reduced resistin mRNA expression. Gastric ghrelin mRNA expression during fasting was increased by a high fat diet. GHS-R antagonists decreased energy intake in lean mice, in mice with diet induced obesity, and in ob/ob obese mice; it also reduced the rate of gastric emptying. Repeated administration of GHS-R antagonist decreased body weight gain and improved glycaemic control in ob/ob obese mice.
Conclusions: Ghrelin appears to be closely related to excess weight gain, adiposity, and insulin resistance, particularly under a high fat diet and in the dynamic stage. Gastric peptide ghrelin and GHS-R may be promising therapeutic targets not only for anorexia-cachexia but also for obesity and type 2 diabetes, which are becoming increasingly prevalent worldwide.
Figures
Comment in
-
Gut and mind.Gut. 2003 Jul;52(7):918-21. doi: 10.1136/gut.52.7.918. Gut. 2003. PMID: 12801943 Free PMC article.
References
-
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. - PubMed
-
- Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci 2001;2:551–60. - PubMed
-
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–13. - PubMed
-
- Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001;120:337–45. - PubMed
-
- Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001;409:194–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
