Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing
- PMID: 12802007
- PMCID: PMC164662
- DOI: 10.1073/pnas.1336920100
Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing
Abstract
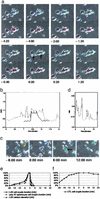
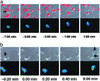
Cytolytic killing is a major effector mechanism in the elimination of virally infected and tumor cells. The innate cytolytic effectors, natural killer (NK) cells, and the adaptive effectors, cytotoxic T cells (CTL), despite differential immune recognition, both use the same lytic mechanism, cytolytic granule release. Using live cell video fluorescence microscopy in various primary cell models of NK cell and CTL killing, we show here that on tight target cell contact, a majority of the NK cells established cytoskeletal polarity required for effective lytic function slowly or incompletely. In contrast, CTLs established cytoskeletal polarity rapidly. In addition, NK cell killing was uniquely sensitive to minor interference with cytoskeletal dynamics. We propose that the stepwise NK cell cytoskeletal polarization constitutes a series of checkpoints in NK cell killing. In addition, the use of more deliberate progression to effector function to compensate for inferior immune recognition specificity provides a mechanistic explanation for how the same effector function can be used in the different functional contexts of the innate and adaptive immune response.
Figures


References
-
- Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197-216. - PubMed
-
- Yokoyama, W. M. (1995) Curr. Opin. Immunol. 7, 110-120. - PubMed
-
- Lanier, L. L. (1998) Annu. Rev. Immunol. 16, 359-393. - PubMed
-
- Moretta, A., Biassoni, R., Bottino, C., Mingari, M. C. & Moretta, L. (2000) Immunol. Today 21, 228-234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

