Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice
- PMID: 12802011
- PMCID: PMC164612
- DOI: 10.1073/pnas.1332608100
Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice
Abstract
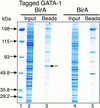
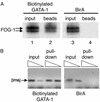
Proteomic approaches require simple and efficient protein purification methodologies that are amenable to high throughput. Biotinylation is an attractive approach for protein complex purification due to the very high affinity of avidin/streptavidin for biotinylated templates. Here, we describe an approach for the single-step purification of transcription factor complex(es) based on specific in vivo biotinylation. We expressed the bacterial BirA biotin ligase in mammalian cells and demonstrated very efficient biotinylation of a hematopoietic transcription factor bearing a small (23-aa) artificial peptide tag. Biotinylation of the tagged transcription factor altered neither the factor's protein interactions or DNA binding properties in vivo nor its subnuclear distribution. Using this approach, we isolated the biotin-tagged transcription factor and at least one other known interacting protein from crude nuclear extracts by direct binding to streptavidin beads. Finally, this method works efficiently in transgenic mice, thus raising the prospect of using biotinylation tagging in protein complex purification directly from animal tissues. Therefore, BirA-mediated biotinylation of tagged proteins provides the basis for the single-step purification of proteins from mammalian cells.
Figures


References
-
- Lamond, A. I. & Mann, M. (1997) Trends Cell Biol. 7, 139–142. - PubMed
-
- Kumar, A. & Snyder, M. (2002) Nature 415, 123–124. - PubMed
-
- Jarvik, J. W. & Telmer, C. A. (1998) Annu. Rev. Genet. 32, 601–618. - PubMed
-
- Rigaut, G., Shevchenko, A., Rutz, B., Wilm, M., Mann, M. & Seraphin, B. (1999) Nat. Biotechnol. 17, 1030–1032. - PubMed
-
- Chapman-Smith, A. & Cronan, J. E., Jr. (1999) Trends Biochem. Sci. 24, 359–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

