Carbohydrate-dependent signaling from the phosphatidylglucoside-based microdomain induces granulocytic differentiation of HL60 cells
- PMID: 12802014
- PMCID: PMC164607
- DOI: 10.1073/pnas.1232503100
Carbohydrate-dependent signaling from the phosphatidylglucoside-based microdomain induces granulocytic differentiation of HL60 cells
Abstract
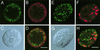
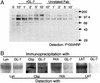
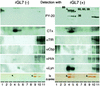
Glycosphingolipids form glycosphingolipid signaling microdomains. Here, we report an unrecognized type of phosphatidylglucoside (PhGlc)-based lipid microdomain in HL60 cells. Treatment of cells with rGL-7, which preferentially reacts with PhGlc, induced differentiation of HL60 cells. This was manifested by the appearance of nitroblue tetrazolium-positive cells together with CD38 expression and c-Myc down-regulation. We determined the molecular mechanisms underlying early stages of signal transduction. rGL-7 treatment induced rapid tyrosine phosphorylation of Src family protein kinases Lyn and Hck. Reduction of endogenous cholesterol after application of methyl-beta-cyclodextrin suppressed rGL-7-stimulated tyrosine phosphorylation. Phosphorylated proteins and PhGlc colocalized in the Triton X-100 insoluble, light buoyant density fraction after sucrose gradient ultracentrifugation of HL60 cell lysates. This suggests PhGlc-based microdomain is involved in GL-7 signaling. Ligation of known components of microdomains, such as sphingomyelin and ganglioside GM1, with corresponding antibodies failed to induce differentiation and tyrosine phosphorylation. These results show that PhGlc constitutes a previously undescribed lipid signaling domain, and the glucose residue of PhGlc is critical for organization of the carbohydrate-dependent signaling domain involved in cellular differentiation of HL60 cells.
Figures




Similar articles
-
Modulation of CD157 expression in multi-lineage myeloid differentiation of promyelocytic cell lines.Eur J Cell Biol. 2000 Oct;79(10):697-706. doi: 10.1078/0171-9335-00099. Eur J Cell Biol. 2000. PMID: 11089918
-
Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation.Mol Cell Biol. 2001 Dec;21(24):8318-28. doi: 10.1128/MCB.21.24.8318-8328.2001. Mol Cell Biol. 2001. PMID: 11713268 Free PMC article.
-
Tetraspanin CD82 controls the association of cholesterol-dependent microdomains with the actin cytoskeleton in T lymphocytes: relevance to co-stimulation.J Cell Sci. 2004 Oct 15;117(Pt 22):5269-82. doi: 10.1242/jcs.01380. Epub 2004 Sep 28. J Cell Sci. 2004. PMID: 15454569
-
Organization and functions of glycolipid-enriched microdomains in phagocytes.Biochim Biophys Acta. 2015 Jan;1851(1):90-7. doi: 10.1016/j.bbalip.2014.06.009. Epub 2014 Jun 23. Biochim Biophys Acta. 2015. PMID: 24968752 Review.
-
Phosphatidylglucoside: a new marker for lipid rafts.Biochim Biophys Acta. 2008 Mar;1780(3):405-9. doi: 10.1016/j.bbagen.2007.08.016. Epub 2007 Sep 6. Biochim Biophys Acta. 2008. PMID: 17933468 Review.
Cited by
-
Lysophosphatidylglucoside/GPR55 signaling promotes foam cell formation in human M2c macrophages.Sci Rep. 2023 Aug 6;13(1):12740. doi: 10.1038/s41598-023-39904-x. Sci Rep. 2023. PMID: 37544935 Free PMC article.
-
Glycolipids: Linchpins in the Organization and Function of Membrane Microdomains.Front Cell Dev Biol. 2020 Oct 29;8:589799. doi: 10.3389/fcell.2020.589799. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195253 Free PMC article. Review.
-
A world of sphingolipids and glycolipids in the brain--novel functions of simple lipids modified with glucose.Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(4):129-43. doi: 10.2183/pjab.88.129. Proc Jpn Acad Ser B Phys Biol Sci. 2012. PMID: 22498977 Free PMC article. Review.
-
Stem cell glycolipids.Neurochem Res. 2011 Sep;36(9):1623-35. doi: 10.1007/s11064-010-0358-1. Epub 2010 Dec 16. Neurochem Res. 2011. PMID: 21161592 Review.
-
Fucoganglioside alpha-fucosyl(alpha-galactosyl)-GM1: a novel member of lipid membrane microdomain components involved in PC12 cell neuritogenesis.Biochem J. 2007 Oct 1;407(1):31-40. doi: 10.1042/BJ20070090. Biochem J. 2007. PMID: 17608628 Free PMC article.
References
-
- Simons, K. & Ikonen, E. (1997) Nature 387, 569–572. - PubMed
-
- Brown, D. A. & London, E. (1998) Annu. Rev. Cell Dev. Biol. 14, 111–135. - PubMed
-
- Brown, D. A. & London, E. (2000) J. Biol. Chem. 275, 17221–17224. - PubMed
-
- Tillack, T. W., Allietta, M., Moran, R. E. & Young, W. W. J. (1983) Biochim. Biophys. Acta 733, 15–24. - PubMed
-
- Okada, Y., Mugnai, G., Bremer, E. G. & Hakomori, S. (1984) Exp. Cell Res. 155, 448–456. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

