Human PIG-U and yeast Cdc91p are the fifth subunit of GPI transamidase that attaches GPI-anchors to proteins
- PMID: 12802054
- PMCID: PMC165076
- DOI: 10.1091/mbc.e02-12-0794
Human PIG-U and yeast Cdc91p are the fifth subunit of GPI transamidase that attaches GPI-anchors to proteins
Abstract
Many eukaryotic proteins are anchored to the cell surface via glycosylphosphatidylinositol (GPI), which is posttranslationally attached to the carboxyl-terminus by GPI transamidase. The mammalian GPI transamidase is a complex of at least four subunits, GPI8, GAA1, PIG-S, and PIG-T. Here, we report Chinese hamster ovary cells representing a new complementation group of GPI-anchored protein-deficient mutants, class U. The class U cells accumulated mature and immature GPI and did not have in vitro GPI transamidase activity. We cloned the gene responsible, termed PIG-U, that encoded a 435-amino-acid hydrophobic protein. The GPI transamidase complex affinity-purified from cells expressing epitope-tagged-GPI8 contained PIG-U and four other known components. Cells lacking PIG-U formed complexes of the four other components normally but had no ability to cleave the GPI attachment signal peptide. Saccharomyces cerevisiae Cdc91p, with 28% amino acid identity to PIG-U, partially restored GPI-anchored proteins on the surface of class U cells. PIG-U and Cdc91p have a functionally important short region with similarity to a region conserved in long-chain fatty acid elongases. Taken together, PIG-U and the yeast orthologue Cdc91p are the fifth component of GPI transamidase that may be involved in the recognition of either the GPI attachment signal or the lipid portion of GPI.
Figures

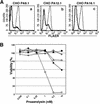

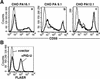




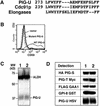
References
-
- Abrami, L., Fivaz, M., Kobayashi, T., Kinoshita, T., Parton, R.G., and van der Goot, F.G. (2001). Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276, 30729–30736. - PubMed
-
- Abrami, L., Fivaz, M., and van der Goot, F.G. (2000). Adventures of a pore-forming toxin at the target cell surface. Trends Microbiol 8, 168–172. - PubMed
-
- Buckley, J.T. (1999). The channel-forming toxin aerolysin. In: The Comprehensive Sourcebook of Bacterial Protein Toxins, ed. J.E. Alouf and J.H. Freer, London: Academic Press, 362–372.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

