Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids
- PMID: 12802055
- PMCID: PMC165077
- DOI: 10.1091/mbc.e02-09-0583
Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids
Abstract
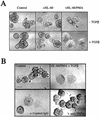
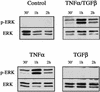
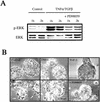
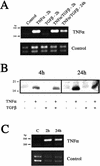
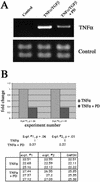
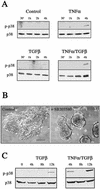
An epithelial-mesenchymal transition (EMT) characterizes the progression of many carcinomas and it is linked to the acquisition of an invasive phenotype. Given that the tumor microenvironment is an active participant in tumor progression, an important issue is whether a reactive stroma can modulate this process. Using a novel EMT model of colon carcinoma spheroids, we demonstrate that their transforming-growth factor-beta1 (TGF-beta)-induced EMT is accelerated dramatically by the presence of activated macrophages, and we identify tumor necrosis factor-alpha (TNF-alpha) as the critical factor produced by macrophages that accelerates the EMT. A synergy of TNF-alpha and TGF-beta signaling promotes a rapid morphological conversion of the highly organized colonic epithelium to dispersed cells with a mesenchymal phenotype, and this process is dependent on enhanced p38 MAPK activity. Moreover, exposure to TNF-alpha stimulates a rapid burst of ERK activation that results in the autocrine production of this cytokine by the tumor cells themselves. These results establish a novel role for the stroma in influencing EMT in colon carcinoma, and they identify a selective advantage to the stromal presence of infiltrating leukocytes in regulating malignant tumor progression.
Figures


References
-
- Arias, A.M. (2001). Epithelial mesenchymal interactions in cancer and development. Cell 105, 425–431. - PubMed
-
- Bakin, A.V., Rinehart, C., Tomlinson, A.K., and Arteaga, C.L. (2002). p38 mitogen-activated kinase is required for TGF beta-mediated fibroblastic transdifferentiation, and cell migration. J. Cell Sci. 115, 3193–3206. - PubMed
-
- Barth, R.J., Jr., Camp, B.J., Martuscello, T.A., Dain, B.J., and Memoli, V.A. (1996). The cytokine microenvironment of human colon carcinoma. Lymphocyte expression of tumor necrosis factor-alpha and interleukin-4 predicts improved survival. Cancer 78, 1168–1178. - PubMed
-
- Bates, R.C., Edwards, N.S., and Yates, J.D. (2000). Spheroids and cell survival. Crit. Rev. Oncol. Hematol. 36, 61–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

