Dual prenylation is required for Rab protein localization and function
- PMID: 12802060
- PMCID: PMC165082
- DOI: 10.1091/mbc.e02-11-0707
Dual prenylation is required for Rab protein localization and function
Abstract
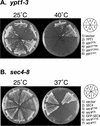
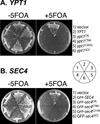
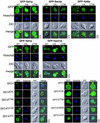
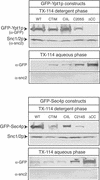
The majority of Rab proteins are posttranslationally modified with two geranylgeranyl lipid moieties that enable their stable association with membranes. In this study, we present evidence to demonstrate that there is a specific lipid requirement for Rab protein localization and function. Substitution of different prenyl anchors on Rab GTPases does not lead to correct function. In the case of YPT1 and SEC4, two essential Rab genes in Saccharomyces cerevisiae, alternative lipid tails cannot support life when present as the sole source of YPT1 and SEC4. Furthermore, our data suggest that double geranyl-geranyl groups are required for Rab proteins to correctly localize to their characteristic organelle membrane. We have identified a factor, Yip1p that specifically binds the di-geranylgeranylated Rab and does not interact with mono-prenylated Rab proteins. This is the first demonstration that the double prenylation modification of Rab proteins is an important feature in the function of this small GTPase family and adds specific prenylation to the already known determinants of Rab localization.
Figures


References
-
- Bai, C., and Elledge, S.J. (1996). Gene identification using the yeast two-hybrid system. Methods Enzymol. 1996, 331–347. - PubMed
-
- Bevis, B.J., and Glick, B.S. (2002). Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat. Biotechnol. 20, 83–87. - PubMed
-
- Black, S.D. (1992). Development of hydrophobicity parameters for prenylated proteins. Biochem. Biophys. Res. Commun. 186, 1437–1442. - PubMed
-
- Bordier, C. (1981). Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256, 1604–1607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

