Ykt6p is a multifunctional yeast R-SNARE that is required for multiple membrane transport pathways to the vacuole
- PMID: 12802061
- PMCID: PMC165083
- DOI: 10.1091/mbc.e02-10-0687
Ykt6p is a multifunctional yeast R-SNARE that is required for multiple membrane transport pathways to the vacuole
Abstract
Intracellular membrane fusion requires that membrane-bound soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins on both vesicle and target membranes form a highly specific complex necessary to bring the membranes close in space. Ykt6p is a yeast R-SNARE protein that has been implicated in retrograde transport to the cis-Golgi compartment. Ykt6p has been also been found to fractionate with vacuole membranes and participate in a vacuolar SNARE complex in homotypic vacuole fusion. To investigate the role of Ykt6p in membrane traffic to the vacuole we generated temperature-sensitive mutations in YKT6. One mutation produces an early Golgi block to secretion, and overexpression of the SNARE protein Sft1p suppresses the growth and secretion defects of this mutation. These results are consistent with Ykt6p and Sft1p participating in a SNARE complex associated with retrograde transport to the cis-Golgi. A second set of mutations in YKT6 specifically affects post-Golgi membrane traffic to the vacuole, and the effects of these mutations are not suppressed by Sft1p overexpression. Defects are seen in carboxypeptidase Y sorting, alkaline phosphatase transport, and aminopeptidase I delivery, and in one mutant, overexpression of the SNARE protein Nyv1p suppresses the alkaline phosphatase transport defect. By mutationally separating early and late requirements for Ykt6p, our findings have revealed that Ykt6p is a R-SNARE protein that functions directly in the three biosynthetic pathways to the vacuole.
Figures








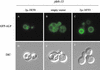
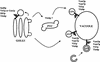
References
-
- Banfield, D.K., Lewis, M.J., and Pelham, H.R. (1995). A SNARE-like protein required for traffic through the Golgi complex. Nature 375, 806–809. - PubMed
-
- Boeke, J.D., LaCroute, F., and Fink, G.R. (1984). A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197, 345–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

