Membrane targeting of Rab GTPases is influenced by the prenylation motif
- PMID: 12802062
- PMCID: PMC165084
- DOI: 10.1091/mbc.e02-10-0639
Membrane targeting of Rab GTPases is influenced by the prenylation motif
Abstract
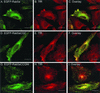
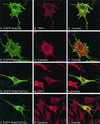
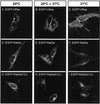
Rab GTPases are regulators of membrane traffic. Rabs specifically associate with target membranes via the attachment of (usually) two geranylgeranyl groups in a reaction involving Rab escort protein and Rab geranylgeranyl transferase. In contrast, related GTPases are singly prenylated by CAAX prenyl transferases. We report that di-geranylgeranyl modification is important for targeting of Rab5a and Rab27a to endosomes and melanosomes, respectively. Transient expression of EGFP-Rab5 mutants containing two prenylatable cysteines (CGC, CC, CCQNI, and CCA) in HeLa cells did not affect endosomal targeting or function, whereas mono-cysteine mutants (CSLG, CVLL, or CVIM) were mistargeted to the endoplasmic reticulum (ER) and were nonfunctional. Similarly, Rab27aCVLL mutant is also mistargeted to the ER and transgenic expression on a Rab27a null background (Rab27aash) did not rescue the coat color phenotype, suggesting that Rab27aCVLL is not functional in vivo. CAAX prenyl transferase inhibition and temperature-shift experiments further suggest that Rabs, singly or doubly modified are recruited to membranes via a Rab escort protein/Rab geranylgeranyl transferase-dependent mechanism that is distinct from the insertion of CAAX-containing GTPases. Finally, we show that both singly and doubly modified Rabs are extracted from membranes by RabGDIalpha and propose that the mistargeting of Rabs to the ER results from loss of targeting information.
Figures









References
-
- Anant, J.S., Desnoyers, L., Machius, M., Demeler, B., Hansen, J.C., Westover, K.D., Deisenhofer, J., and Seabra, M.C. (1998). Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry 37, 12559–12568. - PubMed
-
- Andersson, S., Davis, D.L., Dahlback, H., Jornvall, H., and Russell, D.W. (1989). Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 264, 8222–8229. - PubMed
-
- Andres, D.A., Seabra, M.C., Brown, M.S., Armstrong, S.A., Smeland, T.E., Cremers, F.P., and Goldstein, J.L. (1993). cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell 73, 1091–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

