Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials
- PMID: 12802751
- PMCID: PMC7199898
- DOI: 10.1086/375069
Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials
Abstract
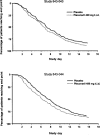
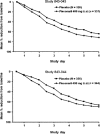
The novel capsid-binding antiviral pleconaril inhibits in vitro replication of most rhinoviruses and enteroviruses. Oral pleconaril treatment was studied in 2 parallel randomized, double-blind, placebo-controlled trials. Among 1363 picornavirus-infected participants (65%) in the studies combined, the median time to alleviation of illness was 1 day shorter for pleconaril recipients than for placebo recipients (P<.001). Cold symptom scores and frequency of picornavirus cultured from nasal mucus specimens were lower among pleconaril recipients by day 2 of treatment. No treatment effects were seen in those without picornavirus infection. Pleconaril was associated with a higher incidence of nausea (6% vs. 4%) and diarrhea (9% vs. 7%) and with small increases in mean serum cholesterol levels and platelet counts, compared with baseline measurements. A subsequent 6-week prophylaxis study found that pleconaril induces cytochrome P-450 3A enzymes, which metabolize a variety of drugs, including ethinyl estradiol. Early pleconaril treatment was well tolerated and significantly reduced the duration and severity of colds due to picornaviruses in adults.
Figures

Comment in
-
Safety and efficacy evaluation of pleconaril for treatment of the common cold.Clin Infect Dis. 2003 Dec 15;37(12):1722. doi: 10.1086/379830. Clin Infect Dis. 2003. PMID: 14689362 No abstract available.
References
-
- Gwaltney JM, Heinz BA. Rhinovirus. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical virology. 2nd ed. Washington, DC: American Society for Microbiology; 2002. pp. 995–1018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

