Supplemental oxygen for the treatment of prethreshold retinopathy of prematurity
- PMID: 12804470
- PMCID: PMC7017219
- DOI: 10.1002/14651858.CD003482
Supplemental oxygen for the treatment of prethreshold retinopathy of prematurity
Abstract
Background: Oxygen has long been implicated in the pathogenesis of retinopathy of prematurity (ROP) and is rigorously monitored in today's neonatal intensive care units. Recent research using a feline model has shown an improvement in ROP outcome of kittens treated with supplemental oxygen. Current treatment for ROP by retinal ablation is not without complications so a non-invasive method of treatment is preferred. The possible effects of long term oxygen supplementation on chronic lung disease, length of hospital stay and growth and development are, however, unknown.
Objectives: To determine whether, in preterm or low birth weight infants with prethreshold ROP, targeting higher as compared to normal transcutaneous oxygen levels or pulse oximetry levels when using supplemental oxygen reduces the progression of ROP to threshold disease and improves visual outcome without any adverse effects.
Search strategy: The standard search strategy of the Cochrane Neonatal Review Group was used. This included searches of the Oxford Database of Perinatal Trials, MEDLINE, previous reviews including cross references, abstracts, conferences and symposia proceedings, expert informants and journal handsearching. An additional literature search of the MEDLINE (1966-June 2002), EMBASE (1980-April 2002), and CINAHL (1982-April 2002) databases was conducted in order to locate any trials in addition to those provided by the Cochrane Controlled Trials Register (CENTRAL/CCTR, The Cochrane Library Issue 2, 2002).
Selection criteria: All randomised or quasi randomised studies comparing higher versus normal target oxygen levels in preterm or low birthweight infants with prethreshold ROP were eligible for inclusion.
Data collection and analysis: The methodological quality of the one eligible trial was assessed independently by two authors for the degree of selection, performance, attrition and detection bias. Data regarding clinical outcomes including progression to threshold ROP, blindness or severe visual impairment, mortality, respiratory morbidities and long term growth were extracted and reviewed independently by two authors. Results were compared and differences resolved as required. Data analysis was conducted according to the standards of the Cochrane Neonatal Review Group.
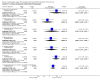
Main results: The one trial included in this review enrolled 649 infants. There was a trend for supplemental oxygen to reduce the progression to threshold ROP, however this did not reach statistical significance (RR 0.84, 95% CI 0.70, 1.02). A subgroup analysis of those infants without plus disease showed significantly fewer infants progressing to threshold ROP in infants treated with supplemental oxygen. However this analysis was not pre-specified so these results should be interpreted with caution. No significant effects were detected on blindness or severe visual function at three months corrected age, mortality, pneumonia, chronic lung disease or weight gain. Adverse pulmonary events were more common in the higher oxygen saturation group and these infants were in hospital and on supplemental oxygen for longer. Longer term visual outcomes were not reported.
Reviewer's conclusions: The results of this systematic review do not show a statistically significant reduction in the rate of progression to threshold ROP with supplemental oxygen treatment, but reveal increased adverse pulmonary sequelae with higher oxygen targeting in this group of preterm infants. Future research needs to be directed towards the question of whether infants without plus disease are more likely to respond to supplemental oxygen therapy than those with plus disease.
Conflict of interest statement
None known.
Figures








References
References to studies included in this review
STOP‐ROP 2000 {published data only}
-
- The STOP‐ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP‐ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000;105:295‐310. - PubMed
References to studies excluded from this review
Gaynon 1997 {published data only}
-
- Gaynon MW, Stevenson DK, Sunshine P, Fleisher BE, Landers MB. Supplemental oxygen may decrease progression of prethreshold disease to threshold retinopathy of prematurity. Journal of Perinatology 1997;17:434‐438. - PubMed
Seiberth 1998 {published data only}
-
- Seiberth V, Linderkamp O, Vardarli I, Jendritza W, Vogele C, Knorz MC. Oxygen therapy in acute retinopathy of prematurity Stage 3. Investigative Ophthalmology and Visual Science 1998;39:S820.
Additional references
Adamkin 1977
-
- Adamkin DH, Shott RJ, Cook LN, Andrews BF. Nonhyperoxic retrolental fibroplasia. Pediatrics 1977;60:828‐30. - PubMed
Aranda 1974
-
- Aranda JV, Sweet AY. Sustained hyperoxemia without cicatricial retrolental fibroplasia. Pediatrics 1974;54:434‐7. - PubMed
Askie 2002
BOOST 2002
-
- Askie LM, Henderson‐Smart DJ, Irwig L, Simpson JM. The effect of differing oxygen saturation targeting ranges on long term growth and development of extremely preterm, oxygen dependent infants: the BOOST Trial [abstract]. Pediatric Research. 2002; Vol. 51:378A.
Chan‐Ling 1995
-
- Chan‐Ling T, Gock B, Stone J. Supplemental oxygen therapy: basis for noninvasive treatment of retinopathy of prematurity. Investigative Ophthalmology and Visual Science 1995;36:1215‐30. - PubMed
Cross 1973
-
- Cross KW. Cost of preventing retrolental fibroplasia. Lancet 1973;2:954‐6. - PubMed
CRYO‐ROP 1988
-
- CRYO‐ROP Cooperative Group. Multicentre trial of cryotherapy for retinopathy of prematurity. Archives of Ophthalmology 1988;106:471‐9. - PubMed
CRYO‐ROP 1994
-
- CRYO‐ROP Cooperative Group. The natural ocular outcome of premature birth and retinopathy: status at 1 year. Archives of Ophthalmology 1994;112:903‐12. - PubMed
Donoghue 2002
-
- Donoghue D, ANZNN. The report of the Australian and New Zealand Neonatal Network 2000. Sydney: ANZNN, 2002:34.
ETROP
-
- Good WV, Hardy RJ. The multicenter study of early treatment for retinopathy of prematurity (ETROP). Ophthalmology 2001;108:1013‐4. - PubMed
Frank 1985
-
- Frank L. Effects of oxygen on the newborn. Federation Proceedings 1985;44:2328‐34. - PubMed
Grieve 1997
-
- Grieve SH, McIntosh N, Laing IA. Comparison of two different pulse oximeters in monitoring preterm infants. Critical Care Medicine 1997;25:2051‐4. - PubMed
ICROP 1984
-
- ICROP. An International Classification of Retinopathy of Prematurity. Pediatrics 1984;74:127‐33. - PubMed
Kaiser 2001
-
- Kaiser RA, Trese MT. Iris atrophy, cataracts and hypertony following peripheral ablation for threshold retinopathy of prematurity. Archives of Ophthalmology 2001;119:615‐7. - PubMed
Laser ROP 1994
-
- The Laser ROP Study Group. Laser therapy for retinopathy of prematurity. Archives of Ophthalmology 1994;112:154‐6. - PubMed
Phelps 1988
-
- Phelps DL. Reduced severity of oxygen‐induced retinopathy in kittens recovered in 28% oxygen. Pediatric Research 1988;24:106‐9. - PubMed
Pierce 1996
-
- Pierce EA, Foley ED, Smith LEH. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Archives of Ophthalmology 1996;114:1219‐54. - PubMed
Reese 1953
-
- Reese AB, King M, Owens WC. A classification of retrolental fibroplasia. American Journal of Ophthalmology 1953;36:1333‐5. - PubMed
Watts 1992
-
- Watts JL. Retinopathy of prematurity. In: Sinclair JC, Bracken MB editor(s). Effective care of the newborn infant. New York: Oxford University Press, 1992:617‐38.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

