The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU
- PMID: 12805211
- PMCID: PMC162142
- DOI: 10.1093/emboj/cdg290
The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU
Abstract
Pseudomonas aeruginosa delivers the toxin ExoU to eukaryotic cells via a type III secretion system. Intoxication with ExoU is associated with lung injury, bacterial dissemination and sepsis in animal model and human infections. To search for ExoU targets in a genetically tractable system, we used controlled expression of the toxin in Saccharomyces cerevisiae. ExoU was cytotoxic for yeast and caused a vacuolar fragmentation phenotype. Inhibitors of human calcium-independent (iPLA(2)) and cytosolic phospholipase A(2) (cPLA(2)) lipase activity reduce the cytotoxicity of ExoU. The catalytic domains of patatin, iPLA(2) and cPLA(2) align or are similar to ExoU sequences. Site-specific mutagenesis of predicted catalytic residues (ExoUS142A or ExoUD344A) eliminated toxicity. ExoU expression in yeast resulted in an accumulation of free palmitic acid, changes in the phospholipid profiles and reduction of radiolabeled neutral lipids. ExoUS142A and ExoUD344A expressed in yeast failed to release palmitic acid. Recombinant ExoU demonstrated lipase activity in vitro, but only in the presence of a yeast extract. From these data we conclude that ExoU is a lipase that requires activation or modification by eukaryotic factors.
Figures


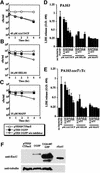

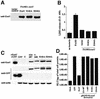
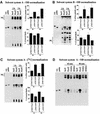
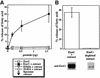
References
-
- Ackermann E.J., Conde-Frieboes,K. and Dennis,E.A. (1995) Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J. Biol. Chem., 270, 445–450. - PubMed
-
- Balsinde J. and Dennis,E.A. (1996) Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem., 271, 6758–6765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

