The thyroid hormone receptor antagonizes CREB-mediated transcription
- PMID: 12805224
- PMCID: PMC162147
- DOI: 10.1093/emboj/cdg295
The thyroid hormone receptor antagonizes CREB-mediated transcription
Abstract
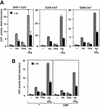
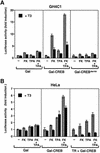
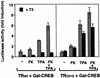
Combinatorial regulation of transcription involves binding of transcription factors to DNA as well as protein-protein interactions between them. In this paper, we demonstrate the existence of a mutual transcriptional antagonism between the thyroid hormone receptor (TR) and the cyclic AMP response element binding protein (CREB), which involves a direct association of both transcription factors. TR inhibits transcriptional activity of CREB and represses activation of cAMP response element (CRE)-containing promoters. TR does not bind to the CRE in vitro, but in vivo the liganded receptor is tethered to the promoter through protein-protein interactions. In turn, expression of CREB reduces TR-dependent transcriptional responses. The association of TR with CREB inhibits the ability of protein kinase A to phosphorylate CREB at Ser133, and leads to a reduction in the ligand-dependent recruitment of the p160 coactivators by TR. These results indicate the existence of a transcriptional cross-talk between CREB and TR signalling pathways, which can have important functional consequences.
Figures



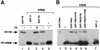

Similar articles
-
Transcriptional activation by thyroid hormone receptor-beta involves chromatin remodeling, histone acetylation, and synergistic stimulation by p300 and steroid receptor coactivators.Mol Endocrinol. 2003 May;17(5):908-22. doi: 10.1210/me.2002-0308. Epub 2003 Feb 13. Mol Endocrinol. 2003. PMID: 12586842
-
Synergistic signaling by corticotropin-releasing hormone and leukemia inhibitory factor bridged by phosphorylated 3',5'-cyclic adenosine monophosphate response element binding protein at the Nur response element (NurRE)-signal transducers and activators of transcription (STAT) element of the proopiomelanocortin promoter.Mol Endocrinol. 2004 Dec;18(12):2997-3010. doi: 10.1210/me.2003-0417. Epub 2004 Aug 19. Mol Endocrinol. 2004. PMID: 15319449
-
Regulation of niemann-pick c1 gene expression by the 3'5'-cyclic adenosine monophosphate pathway in steroidogenic cells.Mol Endocrinol. 2003 Apr;17(4):704-15. doi: 10.1210/me.2002-0093. Epub 2003 Jan 16. Mol Endocrinol. 2003. PMID: 12554781
-
Regulation of somatostatin gene transcription by cyclic adenosine monophosphate.Metabolism. 1996 Aug;45(8 Suppl 1):4-7. doi: 10.1016/s0026-0495(96)90068-2. Metabolism. 1996. PMID: 8769368 Review.
-
Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action.Recent Prog Horm Res. 1997;52:141-64; discussion 164-5. Recent Prog Horm Res. 1997. PMID: 9238851 Review.
Cited by
-
High concentration of estradiol has a negative correlation with free thyroxine during the second trimester of pregnancy.Endocr Connect. 2022 Sep 26;11(10):e220236. doi: 10.1530/EC-22-0236. Print 2022 Oct 1. Endocr Connect. 2022. PMID: 36006849 Free PMC article.
-
Genome-wide binding patterns of thyroid hormone receptor beta.PLoS One. 2014 Feb 18;9(2):e81186. doi: 10.1371/journal.pone.0081186. eCollection 2014. PLoS One. 2014. PMID: 24558356 Free PMC article.
-
The sonic hedgehog-induced type 3 deiodinase facilitates tumorigenesis of basal cell carcinoma by reducing Gli2 inactivation.Endocrinology. 2014 Jun;155(6):2077-88. doi: 10.1210/en.2013-2108. Epub 2014 Apr 2. Endocrinology. 2014. PMID: 24693967 Free PMC article.
-
Thyroid hormone receptor alpha sumoylation modulates white adipose tissue stores.Sci Rep. 2021 Dec 16;11(1):24105. doi: 10.1038/s41598-021-03491-6. Sci Rep. 2021. PMID: 34916557 Free PMC article.
-
Sox9 is involved in the thyroid differentiation program and is regulated by crosstalk between TSH, TGFβ and thyroid transcription factors.Sci Rep. 2022 Feb 9;12(1):2144. doi: 10.1038/s41598-022-06004-1. Sci Rep. 2022. PMID: 35140269 Free PMC article.
References
-
- Aranda A. and Pascual,A. (2001) Nuclear hormone receptors and gene expression. Physiol. Rev., 81, 1269–1304. - PubMed
-
- Arias J., Alberts,A.S., Brindle,P., Claret,F.X., Smeal,T., Karin,M., Feramisco,J. and Montminy,M. (1994) Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature, 370, 226–229. - PubMed
-
- Canettieri G., Morantte,I., Guzman,E., Asahara,H., Herzig,S., Anderson,S.D., Yates,J.R. and Montminy,M. (2003) Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat. Struct. Biol., 10, 175–181. - PubMed
-
- Chakravarti D., LaMorte,V.J., Nelson,M.C., Nakajima,T., Schulman,I.G., Juguilon,H., Montminy,M. and Evans,R.M. (1996) Role of CBP/p300 in nuclear receptor signaling. Nature, 383, 99–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

