Corollary discharge inhibition of ascending auditory neurons in the stridulating cricket
- PMID: 12805311
- PMCID: PMC6740803
- DOI: 10.1523/JNEUROSCI.23-11-04717.2003
Corollary discharge inhibition of ascending auditory neurons in the stridulating cricket
Erratum in
- J Neurosci. 2003 Jul 9;23(14):6161-2
Abstract
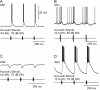
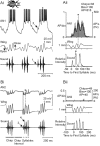
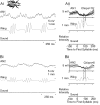
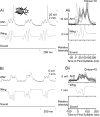
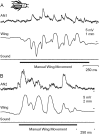
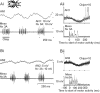
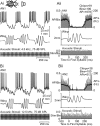
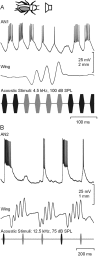
Acoustically communicating animals are able to process external acoustic stimuli despite generating intense sounds during vocalization. We have examined how the crickets' ascending auditory pathway copes with self-generated, intense auditory signals (chirps) during singing (stridulation). We made intracellular recordings from two identified ascending auditory interneurons, ascending neuron 1 (AN1) and ascending neuron 2 (AN2), during pharmacologically elicited sonorous (two-winged), silent (one-winged), and fictive (isolated CNS) stridulation. During sonorous chirps, AN1 responded with bursts of spikes, whereas AN2 was inhibited and rarely spiked. Low-amplitude hyperpolarizing potentials were recorded in AN1 and AN2 during silent chirps. The potentials were also present during fictive chirps. Therefore, they were the result of a centrally generated corollary discharge from the stridulatory motor network. The spiking response of AN1 and AN2 to acoustic stimuli was inhibited during silent and fictive chirps. The maximum period of inhibition occurred in phase with the maximum spiking response to self-generated sound in a sonorously stridulating cricket. In some experiments (30%) depolarizing potentials were recorded during silent chirps. Reafferent feedback elicited by wing movement was probably responsible for the depolarizing potentials. In addition, two other sources of inhibition were present in AN1: (1) IPSPs were elicited by stimulation with 12.5 kHz stimuli and (2) a long-lasting hyperpolarization followed spiking responses to 4.5 kHz stimuli. The hyperpolarization desensitized the response of AN1 to subsequent quieter stimuli. Therefore, the corollary discharge will reduce desensitization by suppressing the response of AN1 to self-generated sounds.
Figures


References
-
- Bell CC ( 1981) An efference copy which is modified by reafferent input. Science 214: 450–453. - PubMed
-
- Bell CC ( 1982) Properties of a modifiable efference copy in an electric fish. J Neurophysiol 47: 1043–1056. - PubMed
-
- Bickmeyer U, Kalmring K, Halex H, Mucke A ( 1992) The bimodal auditory-vibratory system of the thoracic ventral nerve cord in Locusta migratoria (Acrididae, Locustinae, Oedipodini). J Exp Zool 264: 381–394. - PubMed
-
- Blakemore S-J, Wolpert DW, Frith CD ( 1998) Central cancellation of self-produced tickle sensation. Nat Neurosci 1: 635–640. - PubMed
-
- Bodznick D, Montgomery JC, Carey M ( 1999) Adaptive mechanisms in the elasmobranch hindbrain. J Exp Biol 202: 1357–1364. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
