Human theta oscillations related to sensorimotor integration and spatial learning
- PMID: 12805312
- PMCID: PMC6740775
- DOI: 10.1523/JNEUROSCI.23-11-04726.2003
Human theta oscillations related to sensorimotor integration and spatial learning
Abstract
oscillations in the rat hippocampus have been implicated in sensorimotor integration (Bland, 1986), especially during exploratory and wayfinding behavior. We propose that human cortical activity coordinates sensory information with a motor plan to guide wayfinding behavior to known goal locations. To test this hypothesis, we analyzed invasive recordings from epileptic patients while they performed a spatially immersive, virtual taxi driver task. Consistent with this hypothesis, we found oscillations during both exploratory search and goal-seeking behavior and, in particular, during virtual movement, when sensory information and motor planning were both in flux, compared with periods of self-initiated stillness. oscillations had different topographic and spectral characteristics during searching than during goal-seeking, suggesting that different cortical networks exhibit depending on which cognitive functions are driving behavior (spatial learning during exploration vs orienting to a learned representation during goal-seeking). In contrast, oscillations in the beta band appeared to be related to simple motor planning, likely a variant of the Rolandic mu rhythm. These findings suggest that human cortical oscillations act to coordinate sensory and motor brain activity in various brain regions to facilitate exploratory learning and navigational planning.
Figures





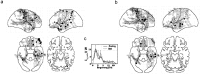
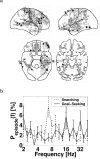

References
-
- Adey WR, Dunlop CW, Hendrix CE ( 1960) Hippocampal slow waves: distribution and phase relationships in the course of approach learning. Arch Neurol 3: 96/74–112/90. - PubMed
-
- Adey WR, Walter DO, Lindsley DF ( 1962) Subthalamic lesions: effects on learned behavior and correlated hippocampal and subcortical slow-wave activity. Arch Neurol 6: 34–47. - PubMed
-
- Berger H ( 1929) Über das Elektroenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrank 87: 527–570.
-
- Berry SD, Thompson RF ( 1978) Prediction of learning rate from the hippocampal electroencephalogram. Science 200: 1298–1300. - PubMed
-
- Bland BH ( 1986) The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26: 1–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
