The 5'-proximal hairpin of turnip yellow mosaic virus RNA: its role in translation and encapsidation
- PMID: 12805444
- PMCID: PMC164824
- DOI: 10.1128/jvi.77.13.7452-7458.2003
The 5'-proximal hairpin of turnip yellow mosaic virus RNA: its role in translation and encapsidation
Abstract
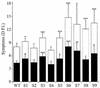
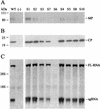
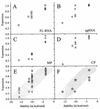
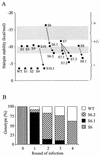
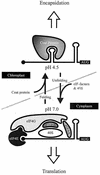
The RNA genome of turnip yellow mosaic virus (TYMV) consists of more than 6,000 nucleotides. During a study of the roles of the two hairpins located in its 90-nucleotide 5' untranslated region, it was observed that stabilization of the 5'-proximal hairpin leads to a delay in the development of symptoms on plants. This delay in symptom development for both locally and systemically infected leaves was found to be dependent on a change in the free energy of the hairpin caused by introduced mutations. A protoplast transfection assay revealed that the accumulation of plus-strand full-length RNA and subgenomic RNA, as well as protein expression levels, was affected by hairpin stability. Stabilization of this hairpin inhibited translation. A model is proposed in which a destabilized 5'-proximal hairpin allows maximal translation of the viral proteins. It is suggested that this hairpin may exist in close proximity to the 5' cap as long as its stability is low enough to enable translation. However, at an acidic pH, the hairpin structure becomes more stable and is functionally transformed into the initiation signal for viral packaging. Slightly acidic conditions can be found in chloroplasts, where TYMV assembly is driven by a low pH generated by active photosynthesis.
Figures


References
-
- Barends, S., H. H. J. Bink, S. H. E. van den Worm, C. W. A. Pleij, and B. Kraal. 2003. Entrapping ribosomes for viral translation: tRNA mimicry as a molecular Trojan horse. Cell 112:123-129. - PubMed
-
- Bozarth, C. S., J. J. Weiland, and T. W. Dreher. 1992. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology 187:124-130. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

