Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice
- PMID: 12805564
- PMCID: PMC166267
- DOI: 10.1073/pnas.1236929100
Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice
Abstract
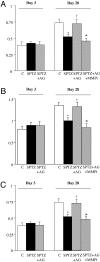
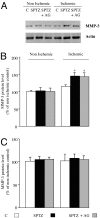
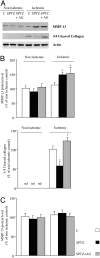
We hypothesized that formation of advanced glycation end products (AGEs) associated with diabetes reduces matrix degradation by metalloproteinases (MMPs) and contributes to the impairment of ischemia-induced angiogenesis. Mice were treated or not with streptozotocin (40 mg/kg) and streptozotocin plus aminoguanidine (AGEs formation blocker, 50 mg/kg). After 8 weeks of treatment, hindlimb ischemia was induced by right femoral artery ligature. Plasma AGE levels were strongly elevated in diabetic mice when compared with control mice (579 +/- 21 versus 47 +/- 4 pmol/ml, respectively; P < 0.01). Treatment with aminoguanidine reduced AGE plasma levels when compared with untreated diabetic mice (P < 0.001). After 28 days of ischemia, ischemic/nonischemic leg angiographic score, capillary density, and laser Doppler skin-perfusion ratios were 1.4-, 1.5-, and 1.4-fold decreased in diabetic mice in reference to controls (P < 0.01). Treatment with aminoguanidine completely normalized ischemia-induced angiogenesis in diabetic mice. We next analyzed the role of proteolysis in AGE formation-induced hampered neovascularization process. After 3 days of ischemia, MMP-2 activity and MMP-3 and MMP-13 protein levels were increased in untreated and aminoguanidine-treated diabetic mice when compared with controls (P < 0.05). Despite this activation of the MMP pathway, collagenolysis was decreased in untreated diabetic mice. Conversely, treatment of diabetic mice with aminoguanidine restored collagenolysis toward levels found in control mice. In conclusion, blockade of AGE formation by aminoguanidine normalizes impaired ischemia-induced angiogenesis in diabetic mice. This effect is probably mediated by restoration of matrix degradation processes that are disturbed as a result of AGE accumulation.
Figures


Similar articles
-
Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: role in ischemia-induced angiogenesis.Circ Res. 2001 Aug 3;89(3):259-64. doi: 10.1161/hh1501.094269. Circ Res. 2001. PMID: 11485976
-
Aminoguanidine inhibits albuminuria, but not the formation of advanced glycation end-products in skin collagen of diabetic rats.Diabetes Res Clin Pract. 1999 Feb;43(2):81-9. doi: 10.1016/s0168-8227(98)00121-1. Diabetes Res Clin Pract. 1999. PMID: 10221660
-
The receptor for advanced glycation end products impairs collateral formation in both diabetic and non-diabetic mice.Lab Invest. 2017 Jan;97(1):34-42. doi: 10.1038/labinvest.2016.113. Epub 2016 Nov 21. Lab Invest. 2017. PMID: 27869797 Free PMC article.
-
Treatment With Small Molecule Inhibitors of Advanced Glycation End-Products Formation and Advanced Glycation End-Products-Mediated Collagen Cross-Linking Promotes Experimental Aortic Aneurysm Progression in Diabetic Mice.J Am Heart Assoc. 2023 May 16;12(10):e028081. doi: 10.1161/JAHA.122.028081. Epub 2023 May 9. J Am Heart Assoc. 2023. PMID: 37158066 Free PMC article.
-
Glycation products and the pathogenesis of diabetic complications.Diabetes Care. 1992 Dec;15(12):1835-43. doi: 10.2337/diacare.15.12.1835. Diabetes Care. 1992. PMID: 1464241 Review.
Cited by
-
Fibronectin-Integrin α5 Signaling in Vascular Complications of Type 1 Diabetes.Diabetes. 2022 Sep 1;71(9):2020-2033. doi: 10.2337/db21-0958. Diabetes. 2022. PMID: 35771994 Free PMC article.
-
Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice.Cardiovasc Diabetol. 2008 May 1;7:13. doi: 10.1186/1475-2840-7-13. Cardiovasc Diabetol. 2008. PMID: 18452614 Free PMC article.
-
Roles of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase in Angiogenesis: Isoform-Specific Effects.Antioxidants (Basel). 2017 Jun 3;6(2):40. doi: 10.3390/antiox6020040. Antioxidants (Basel). 2017. PMID: 28587189 Free PMC article. Review.
-
Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells.Clin Infect Dis. 2008 Sep 1;47(5):634-41. doi: 10.1086/590565. Clin Infect Dis. 2008. PMID: 18652554 Free PMC article.
-
Type 2 Diabetes Mellitus and Latent Tuberculosis Infection Moderately Influence Innate Lymphoid Cell Immune Responses in Uganda.Front Immunol. 2021 Aug 27;12:716819. doi: 10.3389/fimmu.2021.716819. eCollection 2021. Front Immunol. 2021. PMID: 34512639 Free PMC article.
References
-
- Waltenberger, J. (2001) Cardiovasc. Res. 16 554-560. - PubMed
-
- Abaci, A., Oguzhan, A., Kahraman, S., Eryol, N. K., Unal, S., Arinc, H. & Ergin, A. (1999) Circulation 99 2239-2242. - PubMed
-
- Brownlee, M. (2000) Metabolism 49 9-13. - PubMed
-
- Hammes, H. P., Du, X., Edelstein, D., Tagushi, T., Matsumura, T., Ju, Q., Lin, J., Bierhaus, A., Nawroth, P., Hannak, D., et al. (2003) Nat. Med. 9 294-299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

