Regression of established tumors and metastases by potent vascular endothelial growth factor blockade
- PMID: 12805568
- PMCID: PMC164665
- DOI: 10.1073/pnas.1432908100
Regression of established tumors and metastases by potent vascular endothelial growth factor blockade
Abstract
Vascular endothelial growth factor (VEGF) is a critical promoter of blood vessel growth during embryonic development and tumorigenesis. To date, studies of VEGF antagonists have primarily focused on halting progression in models of minimal residual cancer. Consistent with this focus, recent clinical trials suggest that blockade of VEGF may impede cancer progression, presumably by preventing neoangiogenesis. However, VEGF is also a key mediator of endothelial-vascular mural cell interactions, a role that may contribute to the integrity of mature vessels in advanced tumors. Here, we report that high-affinity blockade of VEGF, using the recently described VEGF-Trap, abolishes mature, preexisting vasculature in established xenografts. Eradication of vasculature is followed by marked tumor regression, including regression of lung micrometastases. Thus, the contribution of relatively low levels of VEGF to vessel integrity may be critical to maintenance of even very small tumor masses. Potent blockade of VEGF may provide a new therapeutic option for patients with bulky, metastatic cancers.
Figures
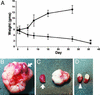
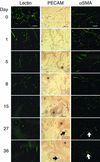

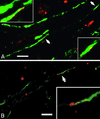
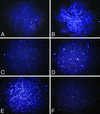
Comment in
-
Building a better Trap.Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8624-5. doi: 10.1073/pnas.1633646100. Epub 2003 Jul 14. Proc Natl Acad Sci U S A. 2003. PMID: 12861079 Free PMC article. No abstract available.
References
-
- Kim, K. J., Li, B., Winer, J., Armanini, M., Gillett, N., Phillips, H. S. & Ferrara, N. (1993) Nature 362, 841-844. - PubMed
-
- Gerber, H. P., Kowalski, J., Sherman, D., Eberhard, D. A. & Ferrara, N. (2000) Cancer Res. 60, 6253-6258. - PubMed
-
- Prewett, M., Huber, J., Li, Y., Santiago, A., O'Connor, W., King, K., Overholser, J., Hooper, A., Pytowski, B., Witte, L., et al. (1999) Cancer Res. 59, 5209-5218. - PubMed
-
- Yang, J. C., Haworth, L., Steinberg, S. M., Rosenberg, S. A. & Novotny, W. (2002) Proc. Am. Soc. Clin. Oncol. 21, 5.
-
- Benjamin, L. E., Hemo, I. & Keshet, E. (1998) Development (Cambridge, U.K.) 125, 1591-1598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

