Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis
- PMID: 12805621
- PMCID: PMC167031
- DOI: 10.1104/pp.103.020123
Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis
Abstract
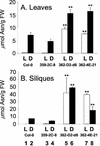
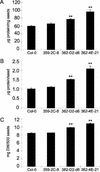
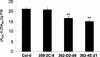
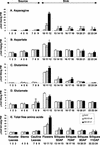
In wild-type Arabidopsis, levels of ASN1 mRNA and asparagine (Asn) are tightly regulated by environmental factors and metabolites. Because Asn serves as an important nitrogen storage and transport compound used to allocate nitrogen resources between source and sink organs, we tested whether overexpression of the major expressed gene for Asn synthetase, ASN1, would lead to changes in nitrogen status in the ultimate storage organ for metabolites-seeds. Transgenic Arabidopsis constitutively overexpressing ASN1 under the cauliflower mosaic virus 35S promoter were constructed (35S-ASN1). In seeds of the 35S-ASN1 lines, three observations support the notion that the nitrogen status was enhanced: (a) elevations of soluble seed protein contents, (b) elevations of total protein contents from acid-hydrolyzed seeds, and (c) higher tolerance of young seedlings when grown on nitrogen-limiting media. Besides quantitative differences, changes in the relative composition of the seed amino acid were also observed. The change in seed nitrogen status was accompanied by an increase of total free amino acids (mainly Asn) allocated to flowers and developing siliques. In 35S-ASN1 lines, sink tissues such as flowers and developing siliques exhibit a higher level of free Asn than source tissues such as leaves and stems, despite significantly higher levels of ASN1 mRNA observed in the source tissues. This was at least partially due to an enhanced transport of Asn from source to sink via the phloem, as demonstrated by the increased levels of Asn in phloem exudates of the 35S-ASN1 plants.
Figures


References
-
- Bechtold N, Pelletier G (1993) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. In J Martinez-Zapater, J Salinas, eds, Arabidopsis Protocols. Humana Press Inc., Totowa, NJ, pp 259-266 - PubMed
-
- Bewley JD, Hempel FD, McCormik S, Zambryski P (2000) Reproductive development. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 988-1043
-
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 - PubMed
-
- Burris RH, Roberts GP (1993) Biological nitrogen fixation. Annu Rev Nutr 13: 317-335 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

