Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-beta production by epithelial cells in mediating antigen-presenting cell function
- PMID: 12807486
- PMCID: PMC1782972
- DOI: 10.1046/j.1365-2567.2003.01670.x
Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-beta production by epithelial cells in mediating antigen-presenting cell function
Abstract
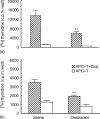
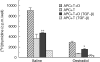
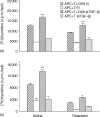
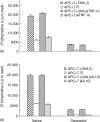
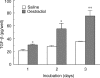
We have previously shown that oestradiol treatment of ovariectomized rats for 3 days inhibits antigen presentation by uterine stromal cells at a time when oestradiol increases the numbers of antigen-presenting cells (APC) in the uterine stroma. In the present study, we found that oestradiol treatment for 1 day is sufficient to inhibit antigen presentation by stromal cells. To define the mechanism(s) of this inhibition, we examined the effect of cytokines and found that exogenous transforming growth factor-beta (TGF-beta) inhibits antigen presentation when stromal cells from saline- but not oestradiol-treated animals are incubated with ovalbumin (OVA)-specific T cells and OVA. In contrast, antigen presentation by uterine epithelial cells was not affected by TGF-beta. In other studies, the acute inhibitory effect of oestradiol (1 day) on stromal antigen presentation is fully reversed when anti-TGF-beta antibody is added to the culture media. When given for 3 days, oestradiol inhibition of antigen presentation is partially reversed by anti-TGF-beta antibody at a time when antibodies to tumour necrosis factor-alpha and interleukin-10 have no effect. To determine whether uterine epithelial cells produce TGF-beta, epithelial cells were grown to confluence on transwell inserts. Our findings indicate that uterine epithelial cells produce biologically active TGF-beta which is preferentially released basolaterally in the direction of underlying stromal cells. When oestradiol is given to ovariectomized rats 1 day before sacrifice, TGF-beta production by epithelial cells increases within 24 hr in culture, relative to saline controls. Taken together, these results suggest that oestradiol inhibition of stromal cell antigen presentation is mediated through the stimulatory effect of oestradiol on TGF-beta production by epithelial cells.
Figures


References
-
- Parr MB, Parr EL. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491. - PubMed
-
- Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract. Comparison of Fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350. - PubMed
-
- Bonatz G, Hansmann ML, Buchholz F, Mettler L, Radzun HJ, Semm K. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int J Gynecol Obstet. 1992;37:29. - PubMed
-
- Yeaman GR, Guyre PM, Fanger MW, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukocyte Biol. 1997;61:427. - PubMed
-
- Wira CR, Kaushic C, Richardson JM. Role of sex hormones and cytokines in regulating the mucosal immune system in the female reproductive tract. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Mucosal Immunology. New York: Academic Press; 1999. p. 1449.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

