Development of the heart: (1) formation of the cardiac chambers and arterial trunks
- PMID: 12807866
- PMCID: PMC1767747
- DOI: 10.1136/heart.89.7.806
Development of the heart: (1) formation of the cardiac chambers and arterial trunks
Figures








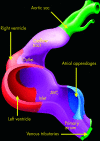

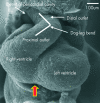



References
-
- Lints TJ, Parsons LM, Hartley L, et al. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 1993;119:419–31. - PubMed
-
- de la Cruz MV, Sanchez-Gómez C, Cayre R. The developmental components of ventricles: their significance in congenital malformations. Cardiol Young 1991;1:123–128. ▸ Maria Victoria de la Cruz was one of the first embryologists to recognise the fact that all parts of the developing heart were not already present at the “straight tube” stage. This paper is a review of her earlier works, and puts the embryological studies into the context of the morphology of congenital cardiac malformations.
-
- Black BL, Olson EN. Control of cardiac development by the MEF2 family of transcription factors. In: Harvey RP, Rosenthal N, eds. Heart development. London: Academic Press, 1999:131–42. ▸ A good chapter in an excellent textbook. As with many of the other chapters, however, the value of the observations concerning advances in molecular biology are devalued to some extent by slavish adherence to concepts of “armchair embryology”.
-
- Knauth A, McCarthy KP, Webb S, et al. Interatrial communication through the mouth of the coronary sinus defect. Cardiol Young 2002;12:364–72. ▸ An important study showing that, from the start of its development, the coronary sinus, derived from the left sinus horn, possessed its own walls discrete from those of the left atrium. - PubMed
-
- Webb S, Kanani M, Anderson RH, et al. Development of the human pulmonary vein and its incorporation in the morphologically left atrium. Cardiol Young 2001;11:632–42. ▸ Conventional wisdom has suggested that the pulmonary vein is derived from the embryonic systemic venous sinus, or “sinus venosus”. This study showed that there is no evidence to support this notion, since the incorporation of the pulmonary vein to the left atrium is a late event in cardiac development. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
