C terminus L-type Ca2+ channel calmodulin-binding domains are 'auto-agonist' ligands in rabbit ventricular myocytes
- PMID: 12807986
- PMCID: PMC2343075
- DOI: 10.1113/jphysiol.2003.043778
C terminus L-type Ca2+ channel calmodulin-binding domains are 'auto-agonist' ligands in rabbit ventricular myocytes
Retraction in
-
Retraction: C terminus L-type Ca2+ channel calmodulin-binding domains are ’auto-agonist’ ligands in rabbit ventricular myocytes.J Physiol. 2015 May 1;593(9):2245. doi: 10.1113/JP270443. J Physiol. 2015. PMID: 26120648 Free PMC article. No abstract available.
Abstract
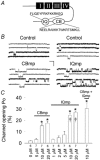
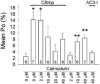
L-type Ca2+ channel C terminus calmodulin (CaM)-binding domains are molecular determinants for Ca(2+)-CaM-dependent increases in L-type Ca2+ current (ICa), and a CaM-binding IQ domain mimetic peptide (IQmp) increases L-type Ca2+ channel current by promoting a gating mode with prolonged openings (mode 2), suggesting the intriguing possibility that CaM-binding domains are 'auto-agonist' signalling molecules. In order to test the breadth of this concept, we studied the effect of a second C terminus CaM-binding domain (CB) mp (CBmp), in conjunction with IQmp, on single L-type Ca2+ channel currents in excised cell membrane patches from rabbit ventricular myocytes. Here we show that both CBmp and IQmp are agonist ligands that non-additively increase L-type Ca2+ channel opening probability (Po) by inducing mode 2 gating. CBmp and IQmp agonist effects were lost under conditions favouring calcification of CaM (Ca(2+)-CaM, 150 nM free Ca2+ and 10-20 microM CaM), but persisted in the presence of CaM (0-20 microM) under conditions adverse to Ca(2+)-CaM (20 mM BAPTA), indicating that CaM-binding domains increase L-type Ca2+ channel Po by a low Ca(2+)-CaM activity mechanism. Increasing Ca(2+)-CaM in the bath (cytosol) reduced the efficacy of CBmp and IQmp signals with Ba2+ as charge carrier, suggesting that CaM binding motifs target a site outside of the pore region. We measured the combined effects of CBmp and Ca(2+)-CaM-dependent protein kinase II (CaMKII) on L-type Ca2+ channels by using an engineered Ca(2+)-CaM-independent form of CaMKII that remains active under low Ca(2+)-CaM conditions, permissive for CBmp signalling. CBmp and CaMKII increased L-type Ca2+ channel Po in a non-additive manner, suggesting that low and high Ca(2+)-CaM-dependent L-type Ca2+ channel facilitation pathways converge upon a common signalling mechanism.
Figures




Comment in
-
Findings of research misconduct.NIH Guide Grants Contracts (Bethesda). 2014 Dec 5:NOT-OD-15-031. NIH Guide Grants Contracts (Bethesda). 2014. PMID: 25528784 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2014 Nov 25;79(227):70187-70188. Fed Regist. 2014. PMID: 27737250 Free PMC article. No abstract available.
References
-
- Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994;75:854–861. - PubMed
-
- Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J Biol Chem. 2001;276:20144–20153. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

