Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts
- PMID: 12808047
- PMCID: PMC194898
- DOI: 10.1091/mbc.e02-11-0729
Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts
Abstract
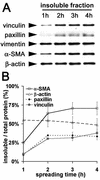
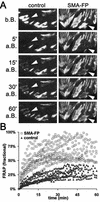
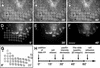
Cultured myofibroblasts are characterized by stress fibers, containing alpha-smooth muscle actin (alpha-SMA) and by supermature focal adhesions (FAs), which are larger than FAs of alpha-SMA-negative fibroblasts. We have investigated the role of alpha-SMA for myofibroblast adhesion and FA maturation. Inverted centrifugation reveals two phases of initial myofibroblast attachment: during the first 2 h of plating microfilament bundles contain essentially cytoplasmic actin and myofibroblast adhesion is similar to that of alpha-SMA-negative fibroblasts. Then, myofibroblasts incorporate alpha-SMA in stress fibers, develop mature FAs and their adhesion capacity is significantly increased. When alpha-SMA expression is induced in 5 d culture by TGFbeta or low serum levels, fibroblast adhesion is further increased correlating with a "supermaturation" of FAs. Treatment of myofibroblasts with alpha-SMA fusion peptide (SMA-FP), which inhibits alpha-SMA-mediated contractile activity, reduces their adhesion to the level of alpha-SMA negative fibroblasts. With the use of flexible micropatterned substrates and EGFP-constructs we show that SMA-FP application leads to a decrease of myofibroblast contraction, shortly followed by disassembly of paxillin- and beta3 integrin-containing FAs; alpha5 integrin distribution is not affected. FRAP of beta3 integrin-EGFP demonstrates an increase of FA protein turnover following SMA-FP treatment. We conclude that the formation and stability of supermature FAs depends on a high alpha-SMA-mediated contractile activity of myofibroblast stress fibers.
Figures





References
-
- Arora, P.D., and McCulloch, C.A. (1994). Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J. Cell. Physiol. 159, 161-175. - PubMed
-
- Balaban, N.Q. et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466-472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

