The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes
- PMID: 12808049
- PMCID: PMC194900
- DOI: 10.1091/mbc.e02-12-0790
The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes
Abstract
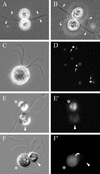
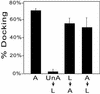
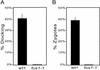
The molecular mechanisms of the defining event in fertilization, gamete fusion, remain poorly understood. The FUS1 gene in the unicellular, biflagellated green alga Chlamydomonas is one of the few sex-specific eukaryotic genes shown by genetic analysis to be essential for gamete fusion during fertilization. In Chlamydomonas, adhesion and fusion of the plasma membranes of activated mt+ and mt- gametes is accomplished via specialized fusion organelles called mating structures. Herein, we identify the endogenous Fus1 protein, test the idea that Fus1 is at the site of fusion, and identify the step in fusion that requires Fus1. Our results show that Fus1 is a approximately 95-kDa protein present on the external surface of both unactivated and activated mt+ gametes. Bioassays indicate that adhesion between mating type plus and mating type minus fusion organelles requires Fus1 and that Fus1 is functional only after gamete activation. Finally, immunofluorescence demonstrates that the Fus1 protein is present as an apical patch on unactivated gametes and redistributes during gamete activation over the entire surface of the microvillous-like activated plus mating structure, the fertilization tubule. Thus, Fus1 is present on mt+ gametes at the site of cell-cell fusion and essential for an early step in the fusion process.
Figures



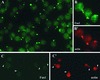
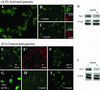
Similar articles
-
The Chlamydomonas mating type plus fertilization tubule, a prototypic cell fusion organelle: isolation, characterization, and in vitro adhesion to mating type minus gametes.J Cell Biol. 1997 Jun 30;137(7):1537-53. doi: 10.1083/jcb.137.7.1537. J Cell Biol. 1997. PMID: 9199169 Free PMC article.
-
A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii.Mol Biol Cell. 1996 Aug;7(8):1235-48. doi: 10.1091/mbc.7.8.1235. Mol Biol Cell. 1996. PMID: 8856667 Free PMC article.
-
Membrane fusion triggers rapid degradation of two gamete-specific, fusion-essential proteins in a membrane block to polygamy in Chlamydomonas.Development. 2010 May;137(9):1473-81. doi: 10.1242/dev.044743. Epub 2010 Mar 24. Development. 2010. PMID: 20335357 Free PMC article.
-
Protein transport and signal transduction during fertilization in chlamydomonas.Traffic. 2003 Jul;4(7):452-9. doi: 10.1034/j.1600-0854.2003.00105.x. Traffic. 2003. PMID: 12795690 Review.
-
Uncovering an ancestral green ménage à trois: Contributions of Chlamydomonas to the discovery of a broadly conserved triad of plant fertilization proteins.Curr Opin Plant Biol. 2022 Oct;69:102275. doi: 10.1016/j.pbi.2022.102275. Epub 2022 Aug 22. Curr Opin Plant Biol. 2022. PMID: 36007296 Free PMC article. Review.
Cited by
-
Anisogamy evolved with a reduced sex-determining region in volvocine green algae.Commun Biol. 2018 Mar 8;1:17. doi: 10.1038/s42003-018-0019-5. eCollection 2018. Commun Biol. 2018. PMID: 30271904 Free PMC article.
-
The functional domain of GCS1-based gamete fusion resides in the amino terminus in plant and parasite species.PLoS One. 2010 Dec 31;5(12):e15957. doi: 10.1371/journal.pone.0015957. PLoS One. 2010. PMID: 21209845 Free PMC article.
-
Fusexins, HAP2/GCS1 and Evolution of Gamete Fusion.Front Cell Dev Biol. 2022 Jan 10;9:824024. doi: 10.3389/fcell.2021.824024. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35083224 Free PMC article. Review.
-
Sexual reproduction and sex determination in green algae.J Plant Res. 2017 May;130(3):423-431. doi: 10.1007/s10265-017-0908-6. Epub 2017 Feb 10. J Plant Res. 2017. PMID: 28188480 Review.
-
The elusive actin cytoskeleton of a green alga expressing both conventional and divergent actins.Mol Biol Cell. 2019 Oct 15;30(22):2827-2837. doi: 10.1091/mbc.E19-03-0141. Epub 2019 Sep 18. Mol Biol Cell. 2019. PMID: 31532705 Free PMC article.
References
-
- Adair, W.S. (1985). Characterization of Chlamydomonas sexual agglutinins. J. Cell Sci. Suppl. 2, 233-260. - PubMed
-
- Buchanan, M.J., Imam, S.H., Eskue, W.A., and Snell, W.J. (1989). Activation of the cell wall degrading protease, lysin, during sexual signalling in Chlamydomonas: the enzyme is stored as an inactive, higher relative molecular mass precursor in the periplasm. J. Cell Biol. 108, 199-207. - PMC - PubMed
-
- Buchanan, M.J., and Snell, W.J. (1988). Biochemical studies on lysin, a cell wall degrading enzyme released during fertilization in Chlamydomonas. Exp. Cell Res. 179, 181-193. - PubMed
-
- Dimitrov, D.S. (2000). Cell biology of virus entry. Cell 101, 697-702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

