Loss of Sin3/Rpd3 histone deacetylase restores the DNA damage response in checkpoint-deficient strains of Saccharomyces cerevisiae
- PMID: 12808094
- PMCID: PMC164854
- DOI: 10.1128/MCB.23.13.4522-4531.2003
Loss of Sin3/Rpd3 histone deacetylase restores the DNA damage response in checkpoint-deficient strains of Saccharomyces cerevisiae
Abstract
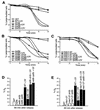
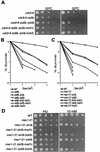
We previously reported that expression of the human forkhead/winged helix transcription factor, CHES1 (checkpoint suppressor 1; FOXN3), suppresses sensitivity to DNA damage and restores damage-induced G(2)/M arrest in checkpoint-deficient strains of Saccharomyces cerevisiae. We find that a functional glutathione S-transferase-Ches1 fusion protein binds in vivo to Sin3, a component of the S. cerevisiae Sin3/Rpd3 histone deacetylase complex. Checkpoint mutant strains with SIN3 deleted show increased resistance to UV irradiation, which is not further enhanced by CHES1 expression. Conversely, overexpression of SIN3 blocks the Ches1-mediated G(2)/M delay in response to DNA damage, which is consistent with Ches1 acting by inhibiting the Sin3/Rpd3 complex. Deletion of either SIN3 or RPD3 in rad9 or mec1 checkpoint mutant strains suppresses sensitivity to replication blocks and DNA damage resulting from Cdc9 ligase deficiency and UV irradiation. SIN3 or RPD3 deletions also restored G(2)/M arrest after DNA damage without concomitant Rad53 phosphorylation in mec1 mutant strains. This DNA damage response is absent in mad1 spindle checkpoint mutants. These data suggest that modulation of chromatin structure may regulate checkpoint responses in S. cerevisiae. Inhibition of histone deacetylation results in a DNA damage checkpoint response mediated by the spindle checkpoint pathway that compensates for loss of the primary DNA damage checkpoint pathway.
Figures



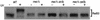
References
-
- Amon, A. 1999. The spindle checkpoint. Curr. Opin. Genet. Dev. 9:69-75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
