Transcriptional program of apoptosis induction following interleukin 2 deprivation: identification of RC3, a calcium/calmodulin binding protein, as a novel proapoptotic factor
- PMID: 12808095
- PMCID: PMC164849
- DOI: 10.1128/MCB.23.13.4532-4541.2003
Transcriptional program of apoptosis induction following interleukin 2 deprivation: identification of RC3, a calcium/calmodulin binding protein, as a novel proapoptotic factor
Abstract
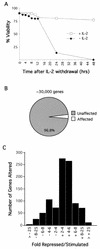
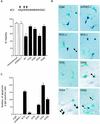
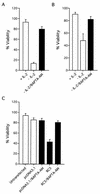
Apoptosis of mature T lymphocytes preserves immune system homeostasis by counteracting transient increases in T-cell number. This process is regulated, at least in part, by the cytokine interleukin 2 (IL-2): T cells deprived of IL-2 undergo apoptosis. The mechanism of apoptosis induction by IL-2 deprivation remains to be determined but is known to require RNA synthesis, implying the existence of transcriptionally activated genes whose products induce cell death. To identify such genes, we have performed expression profiling in IL-2-dependent T cells following cytokine deprivation. Our results reveal an intricate transcriptional program entailing the induction of known proapoptotic factors and the simultaneous repression of known antiapoptotic factors. Surprisingly, one gene whose transcription substantially increased was RC3 (also called neurogranin), which encodes a calmodulin binding protein thought to be a neural-specific factor involved in learning and memory. We show that ectopic expression of RC3 in IL-2-dependent T cells increases the intracellular Ca(2+) concentration and induces apoptosis even in the presence of cytokine. Buffering the Ca(2+) increase with the cytoplasmic Ca(2+) chelator BAPTA-AM [1,2-bis(2-aminophenoxy)ethane-N,N,N1,N-tetraacetic acid] blocks RC3-induced apoptosis, indicating that the rise in intracellular Ca(2+) is required for apoptotic death. RC3 mutants unable to bind calmodulin fail to increase intracellular Ca(2+) levels and to induce apoptosis. Based upon these results, we propose that IL-2 deprivation raises the level of RC3 and other apoptotic factors, which induce apoptosis by increasing the intracellular Ca(2+) concentration.
Figures




References
-
- Baffy, G., T. Miyashita, J. R. Williamson, and J. C. Reed. 1993. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J. Biol. Chem. 268:6511-6519. - PubMed
-
- Bahler, M., and A. Rhoads. 2002. Calmodulin signaling via the IQ motif. FEBS Lett. 513:107-113. - PubMed
-
- Baudier, J., J. C. Deloulme, A. Van Dorsselaer, D. Black, and H. W. Matthes. 1991. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J. Biol. Chem. 266:229-237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
