The Bcr kinase downregulates Ras signaling by phosphorylating AF-6 and binding to its PDZ domain
- PMID: 12808105
- PMCID: PMC164848
- DOI: 10.1128/MCB.23.13.4663-4672.2003
The Bcr kinase downregulates Ras signaling by phosphorylating AF-6 and binding to its PDZ domain
Abstract
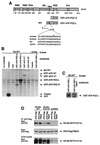
The protein kinase Bcr is a negative regulator of cell proliferation and oncogenic transformation. We identified Bcr as a ligand for the PDZ domain of the cell junction and Ras-interacting protein AF-6. The Bcr kinase phosphorylates AF-6, which subsequently allows efficient binding of Bcr to AF-6, showing that the Bcr kinase is a regulator of the PDZ domain-ligand interaction. Bcr and AF-6 colocalize in epithelial cells at the plasma membrane. In addition, Bcr, AF-6, and Ras form a trimeric complex. Bcr increases the affinity of AF-6 to Ras, and a mutant of AF-6 that lacks a specific phosphorylation site for Bcr shows a reduced binding to Ras. Wild-type Bcr, but not Bcr mutants defective in binding to AF-6, interferes with the Ras-dependent stimulation of the Raf/MEK/ERK pathway. Since AF-6 binds to Bcr via its PDZ domain and to Ras via its Ras-binding domain, we propose that AF-6 functions as a scaffold-like protein that links Bcr and Ras to cellular junctions. We suggest that this trimeric complex is involved in downregulation of Ras-mediated signaling at sites of cell-cell contact to maintain cells in a nonproliferating state.
Figures








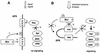
References
-
- Boguski, M. S., and F. McCormick. 1993. Proteins regulating Ras and its relatives. Nature 366:643-654. - PubMed
-
- Buchert, M., S. Schneider, V. Meskenaite, M. T. Adams, E. Canaani, T. Baechi, K. Moelling, and C. M. Hovens. 1999. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell-cell contact in the brain. J. Cell Biol. 144:361-371. - PMC - PubMed
-
- Durfee, T., K. Becherer, P. L. Chen, S. H. Yeh, Y. Yang, A. E. Kilburn, W. H. Lee, and S. J. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7:555-569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
