Inducible nitric oxide synthase expression inhibition by adenovirus E1A
- PMID: 12808130
- PMCID: PMC164663
- DOI: 10.1073/pnas.1337185100
Inducible nitric oxide synthase expression inhibition by adenovirus E1A
Abstract
Nitric oxide (NO) is an antiviral effector of the innate immune system. Viruses that can interfere with NO synthesis may be able to replicate more rapidly than viruses that cannot limit NO synthesis. We show that the adenovirus E1A protein inhibits NO production by decreasing expression of the inducible NO synthase (NOS2). The amino-terminal portion of E1A decreases transactivation of the NOS2 5'-flanking region, limiting the DNA binding activity of NF-kappaB and inhibiting NOS2 expression. E1A is thus able to deactivate a critical component of the host defense against viral infection. Viral inhibition of NO production is a mechanism that may enable certain viruses to evade the host innate immune system.
Figures



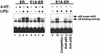

References
-
- Shenk, T. (1996) in Fields Virology, eds. Fields, B., Knipe, D. & Howley, P. (Lippincott–Raven, Philadelphia), Vol. 2, pp. 2111-2148.
-
- Mahr, J. A. & Gooding, L. R. (1999) Immunol. Rev. 168, 121-130. - PubMed
-
- Wold, W. S., Doronin, K., Toth, K., Kuppuswamy, M., Lichtenstein, D. L. & Tollefson, A. E. (1999) Curr. Opin. Immunol. 11, 380-386. - PubMed
-
- Lukashok, S. A. & Horwitz, M. S. (1998) Curr. Clin. Top. Infect. Dis. 18, 286-305. - PubMed
-
- Thimmappaya, B., Weinberger, C., Schneider, R. J. & Shenk, T. (1982) Cell 31, 543-551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

