Expression of utrophin A mRNA correlates with the oxidative capacity of skeletal muscle fiber types and is regulated by calcineurin/NFAT signaling
- PMID: 12808150
- PMCID: PMC164666
- DOI: 10.1073/pnas.0932671100
Expression of utrophin A mRNA correlates with the oxidative capacity of skeletal muscle fiber types and is regulated by calcineurin/NFAT signaling
Abstract
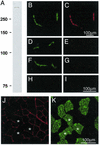
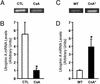
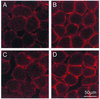
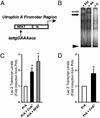
Utrophin levels have recently been shown to be more abundant in slow vs. fast muscles, but the nature of the molecular events underlying this difference remains to be fully elucidated. Here, we determined whether this difference is due to the expression of utrophin A or B, and examined whether transcriptional regulatory mechanisms are also involved. Immunofluorescence experiments revealed that slower fibers contain significantly more utrophin A in extrasynaptic regions as compared with fast fibers. Single-fiber RT-PCR analysis demonstrated that expression of utrophin A transcripts correlates with the oxidative capacity of muscle fibers, with cells expressing myosin heavy chain I and IIa demonstrating the highest levels. Functional muscle overload, which stimulates expression of a slower, more oxidative phenotype, induced a significant increase in utrophin A mRNA levels. Because calcineurin has been implicated in controlling this slower, high oxidative myofiber program, we examined expression of utrophin A transcripts in muscles having altered calcineurin activity. Calcineurin inhibition resulted in an 80% decrease in utrophin A mRNA levels. Conversely, muscles from transgenic mice expressing an active form of calcineurin displayed higher levels of utrophin A transcripts. Electrophoretic mobility shift and supershift assays revealed the presence of a nuclear factor of activated T cells (NFAT) binding site in the utrophin A promoter. Transfection and direct gene transfer studies showed that active forms of calcineurin or nuclear NFATc1 transactivate the utrophin A promoter. Together, these results indicate that expression of utrophin A is related to the oxidative capacity of muscle fibers, and implicate calcineurin and its effector NFAT in this mechanism.
Figures

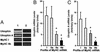
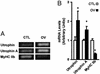
Similar articles
-
The calcineurin-NFAT pathway and muscle fiber-type gene expression.Am J Physiol Cell Physiol. 2000 Oct;279(4):C915-24. doi: 10.1152/ajpcell.2000.279.4.C915. Am J Physiol Cell Physiol. 2000. PMID: 11003571
-
Calcineurin-NFAT signaling, together with GABP and peroxisome PGC-1{alpha}, drives utrophin gene expression at the neuromuscular junction.Am J Physiol Cell Physiol. 2005 Oct;289(4):C908-17. doi: 10.1152/ajpcell.00196.2005. Epub 2005 Jun 1. Am J Physiol Cell Physiol. 2005. PMID: 15930144
-
Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice.Hum Mol Genet. 2004 Feb 15;13(4):379-88. doi: 10.1093/hmg/ddh037. Epub 2003 Dec 17. Hum Mol Genet. 2004. PMID: 14681302
-
Multiple regulatory events controlling the expression and localization of utrophin in skeletal muscle fibers: insights into a therapeutic strategy for Duchenne muscular dystrophy.J Physiol Paris. 2002 Jan-Mar;96(1-2):31-42. doi: 10.1016/s0928-4257(01)00078-x. J Physiol Paris. 2002. PMID: 11755781 Review.
-
Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development.Dev Biol. 2004 Feb 1;266(1):1-16. doi: 10.1016/j.ydbio.2003.10.008. Dev Biol. 2004. PMID: 14729474 Review.
Cited by
-
The effects of neurogranin knockdown on SERCA pump efficiency in soleus muscles of female mice fed a high fat diet.Front Endocrinol (Lausanne). 2022 Aug 22;13:957182. doi: 10.3389/fendo.2022.957182. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072929 Free PMC article.
-
NF-kappaB mediates the transcription of mouse calsarcin-1 gene, but not calsarcin-2, in C2C12 cells.BMC Mol Biol. 2007 Mar 6;8:19. doi: 10.1186/1471-2199-8-19. BMC Mol Biol. 2007. PMID: 17341303 Free PMC article.
-
Activated calcineurin ameliorates contraction-induced injury to skeletal muscles of mdx dystrophic mice.J Physiol. 2006 Sep 1;575(Pt 2):645-56. doi: 10.1113/jphysiol.2006.108472. Epub 2006 Jun 22. J Physiol. 2006. PMID: 16793906 Free PMC article.
-
Intronic regulation of matrix metalloproteinase-2 revealed by in vivo transcriptional analysis in ischemia.Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16345-50. doi: 10.1073/pnas.0508085102. Epub 2005 Oct 28. Proc Natl Acad Sci U S A. 2005. PMID: 16258061 Free PMC article.
-
Sarcolipin deletion in mdx mice impairs calcineurin signalling and worsens dystrophic pathology.Hum Mol Genet. 2018 Dec 1;27(23):4094-4102. doi: 10.1093/hmg/ddy302. Hum Mol Genet. 2018. PMID: 30137316 Free PMC article.
References
-
- Krag, T. O., Gyrd-Hansen, M. & Khurana, T. S. (2001) Acta Physiol. Scand. 171, 349-358. - PubMed
-
- Blake, D. J., Weir, A., Newey, S. E. & Davies, K. E. (2002) Physiol. Rev. 82, 291-329. - PubMed
-
- Jasmin, B. J., Angus, L. M., Belanger, G., Chakkalakal, J. V., Gramolini, A. O., Lunde, J. A., Stocksley, M. A. & Thompson, J. (2002) J. Physiol. (Paris) 96, 31-42. - PubMed
-
- Jasmin, B. J., Alameddine, H., Lunde, J. A., Stetzkowski-Marden, F., Collin, H., Tinsley, J. M., Davies, K. E., Tome, F. M., Parry, D. J. & Cartaud, J. (1995) FEBS Lett. 374, 393-398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

