Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis
- PMID: 12808151
- PMCID: PMC166246
- DOI: 10.1073/pnas.0932636100
Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis
Abstract
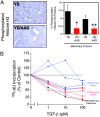
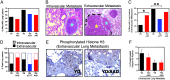
The influence of transforming growth factor beta (TGF-beta) signaling on Neu-induced mammary tumorigenesis and metastasis was examined with transgenic mouse models. We generated mice expressing an activated TGF-beta type I receptor or dominant negative TGF-beta type II receptor under control of the mouse mammary tumor virus promoter. When crossed with mice expressing activated forms of the Neu receptor tyrosine kinase that selectively couple to the Grb2 or Shc signaling pathways the activated type I receptor increased the latency of mammary tumor formation but also enhanced the frequency of extravascular lung metastasis. Conversely, expression of the dominant negative type II receptor decreased the latency of Neu-induced mammary tumor formation while significantly reducing the incidence of extravascular lung metastases. These observations argue that TGF-beta can promote the formation of lung metastases while impairing Neu-induced tumor growth and suggest that extravasation of breast cancer cells from pulmonary vessels is a point of action of TGF-beta in the metastatic process.
Figures



Comment in
-
The two faces of transforming growth factor beta in carcinogenesis.Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8621-3. doi: 10.1073/pnas.1633291100. Epub 2003 Jul 14. Proc Natl Acad Sci U S A. 2003. PMID: 12861075 Free PMC article. Review. No abstract available.
References
-
- Massague, J., Blain, S. W. & Lo, R. S. (2000) Cell 103 295-309. - PubMed
-
- Gold, L. I. (1999) Crit. Rev. Oncog. 10 303-360. - PubMed
-
- Oft, M., Peli, J., Rudaz, C., Schwarz, H., Beug, H. & Reichmann, E. (1996) Genes Dev. 10 2462-2477. - PubMed
-
- Portella, G., Cumming, S. A., Liddell, J., Cui, W., Ireland, H., Akhurst, R. J. & Balmain, A. (1998) Cell Growth Differ. 9 393-404. - PubMed
-
- Gorsch, S. M., Memoli, V. A., Stukel, T. A., Gold, L. I. & Arrick, B. A. (1992) Cancer Res. 52 6949-6952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

