Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice
- PMID: 12808468
- PMCID: PMC2885907
- DOI: 10.1038/nature01761
Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice
Abstract
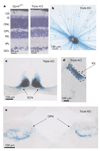
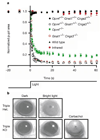
In the mammalian retina, besides the conventional rod-cone system, a melanopsin-associated photoreceptive system exists that conveys photic information for accessory visual functions such as pupillary light reflex and circadian photo-entrainment. On ablation of the melanopsin gene, retinal ganglion cells that normally express melanopsin are no longer intrinsically photosensitive. Furthermore, pupil reflex, light-induced phase delays of the circadian clock and period lengthening of the circadian rhythm in constant light are all partially impaired. Here, we investigated whether additional photoreceptive systems participate in these responses. Using mice lacking rods and cones, we measured the action spectrum for phase-shifting the circadian rhythm of locomotor behaviour. This spectrum matches that for the pupillary light reflex in mice of the same genotype, and that for the intrinsic photosensitivity of the melanopsin-expressing retinal ganglion cells. We have also generated mice lacking melanopsin coupled with disabled rod and cone phototransduction mechanisms. These animals have an intact retina but fail to show any significant pupil reflex, to entrain to light/dark cycles, and to show any masking response to light. Thus, the rod-cone and melanopsin systems together seem to provide all of the photic input for these accessory visual functions.
Figures
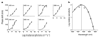


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

