Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection
- PMID: 12810686
- PMCID: PMC2193956
- DOI: 10.1084/jem.20030239
Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection
Abstract
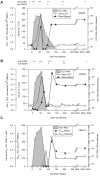
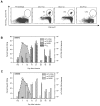
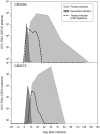
Few hepatitis C virus (HCV) infections resolve spontaneously but those that do appear to afford protective immunity. Second infections are usually shorter in duration and are less likely to persist but mechanisms of virus control in immune individuals have not been identified. In this study we investigated whether memory helper and/or cytotoxic T lymphocytes provide protection in chimpanzees serially reinfected with the virus. Clearance of the first infection took 3-4 mo and coincided with the delayed onset of CD4+ and CD8+ T cell responses. High frequencies of memory T cells targeting multiple HCV proteins were stable over 7 yr of follow-up. Animals were infected for a second time to assess the protective role of memory T cells. In contrast to the prolonged course of the first infection, viremia was terminated within 14 d. Control of this second infection was kinetically linked to rapid acquisition of virus-specific cytolytic activity by liver resident CD8+ T cells and expansion of memory CD4+ and CD8+ T cells in blood. The importance of memory CD8+ T cells in control of HCV infection was confirmed by antibody-mediated depletion of this lymphocyte subset before a third infection. Virus replication was prolonged despite the presence of memory CD4+ T helper cells primed by the two prior infections and was not terminated until HCV-specific CD8+ T cells recovered in the liver. These experiments demonstrate an essential role for memory CD8+ T cells in long-term protection from chronic hepatitis C.
Figures



References
-
- Lauer, G.M., and B.D. Walker. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41–52. - PubMed
-
- Cooper, S., A.L. Erickson, E.J. Adams, J. Kansopon, A.J. Weiner, D.Y. Chien, M. Houghton, P. Parham, and C.M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity. 10:439–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

