Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels
- PMID: 12810850
- PMCID: PMC2234471
- DOI: 10.1085/jgp.200208783
Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels
Abstract
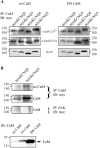
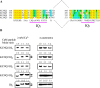
To quantify the modulation of KCNQ2/3 current by [Ca2+]i and to test if calmodulin (CaM) mediates this action, simultaneous whole-cell recording and Ca2+ imaging was performed on CHO cells expressing KCNQ2/3 channels, either alone, or together with wild-type (wt) CaM, or dominant-negative (DN) CaM. We varied [Ca2+]i from <10 to >400 nM with ionomycin (5 microM) added to either a 2 mM Ca2+, or EGTA-buffered Ca2+-free, solution. Coexpression of wt CaM made KCNQ2/3 currents highly sensitive to [Ca2+]i (IC50 70 +/- 20 nM, max inhibition 73%, n = 10). However, coexpression of DN CaM rendered KCNQ2/3 currents largely [Ca2+]i insensitive (max inhibition 8 +/- 3%, n = 10). In cells without cotransfected CaM, the Ca2+ sensitivity was variable but generally weak. [Ca2+]i modulation of M current in superior cervical ganglion (SCG) neurons followed the same pattern as in CHO cells expressed with KCNQ2/3 and wt CaM, suggesting that endogenous M current is also highly sensitive to [Ca2+]i. Coimmunoprecipitations showed binding of CaM to KCNQ2-5 that was similar in the presence of 5 mM Ca2+ or 5 mM EGTA. Gel-shift analyses suggested Ca2+-dependent CaM binding to an "IQ-like" motif present in the carboxy terminus of KCNQ2-5. We tested whether bradykinin modulation of M current in SCG neurons uses CaM. Wt or DN CaM was exogenously expressed in SCG cells using pseudovirions or the biolistic "gene gun." Using both methods, expression of both wt CaM and DN CaM strongly reduced bradykinin inhibition of M current, but for all groups muscarinic inhibition was unaffected. Cells expressed with wt CaM had strongly reduced tonic current amplitudes as well. We observed similar [Ca2+]i rises by bradykinin in all the groups of cells, indicating that CaM did not affect Ca2+ release from stores. We conclude that M-type currents are highly sensitive to [Ca2+]i and that calmodulin acts as their Ca2+ sensor.
Figures



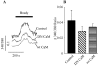
References
-
- Bernheim, L., D.J. Beech, and B. Hille. 1991. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 6:859–867. - PubMed
-
- Bofill-Cardona, E., N. Vartian, C. Nanoff, M. Freissmuth, and S. Boehm. 2000. Two different signaling mechanisms involved in the excitation of rat sympathetic neurons by uridine nucleotides. Mol. Pharmacol. 57:1165–1172. - PubMed
-
- Brown, D.A., and P.R. Adams. 1980. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 283:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

