Exacerbation of experimental autoimmune encephalomyelitis in rodents infected with murine gammaherpesvirus-68
- PMID: 12811845
- PMCID: PMC7163599
- DOI: 10.1002/eji.200323148
Exacerbation of experimental autoimmune encephalomyelitis in rodents infected with murine gammaherpesvirus-68
Abstract
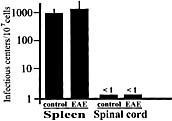
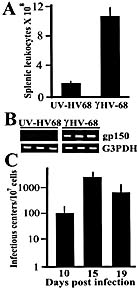
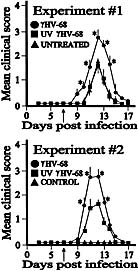
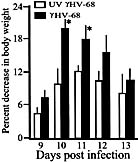
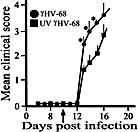
Viral infections have long been suspected to play a role in the pathogenesis of multiple sclerosis. In the present study, two different rodent models of experimental autoimmune encephalomyelitis (EAE) were used to demonstrate the ability of murine gammaherpesvirus-68 (gammaHV-68) to exacerbate development of neurological symptoms. SJL mice received UV-inactivated gammaHV-68 or intranasalgammaHV-68, followed by immunization against proteolipid-protein peptide 139-151. Infected mice became moribund within 10 days post-immunization, whereas mice exposed to UV-inactivated gammaHV-68 recovered. In the second model, Lewis rats were exposed to UV-inactivated gammaHV-68 or to gammaHV-68, followed by passive transfer of encephalitogenic T lymphocytes specific for myelin basic protein. Consistently, infected rats had higher clinical scores, and this result was observed during acute or latent gammaHV-68 infection. It is unlikely that this gammaHV-68-induced exacerbation was due to significant viral replication within the central nervous system since nested PCR, viral plaque assays, and infectious-centers assays demonstrated no detectable virus in spinal cords or brains of infected rodents undergoing EAE. Taken together, these studies demonstrate increased clinical symptoms of EAE in rodents infected by a gammaherpesvirus that has a limited ability to invade the central nervous system.
Figures




References
-
- von Herrath, M. G., Obstacles to identifying viruses that cause autoimmune disease. J. Neuroimmunol. 2000. 107: 154–160. - PubMed
-
- Hickey, W. F., The pathology of multiple sclerosis: a historical perspective. J. Neuroimmunol. 1999. 98: 37–44. - PubMed
-
- Granieri, E., Exogeneous factors in the aetiology of multiple sclerosis. J. Neurovirol. 2000. 6: S141–S146. - PubMed
-
- Lauer, K., The risk of multiple sclerosis in the U.S.A. in relation to sociogeographic features: a factor‐analytic study. J. Clin. Epidemiol. 1994. 47: 43–48. - PubMed
-
- Gale, C. R. and Martyn, C. N., Migrant studies in multiple sclerosis. Prog. Neurobiol. 1995. 47: 425–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

