Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease
- PMID: 12813018
- PMCID: PMC161418
- DOI: 10.1172/JCI16303
Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease
Abstract
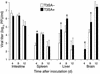
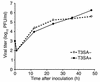
Infection of neonatal mice with some reovirus strains produces a disease similar to infantile biliary atresia, but previous attempts to correlate reovirus infection with this disease have yielded conflicting results. We used isogenic reovirus strains T3SA- and T3SA+, which differ solely in the capacity to bind sialic acid as a coreceptor, to define the role of sialic acid in reovirus encephalitis and biliary tract infection in mice. Growth in the intestine was equivalent for both strains following peroral inoculation. However, T3SA+ spread more rapidly from the intestine to distant sites and replicated to higher titers in spleen, liver, and brain. Strikingly, mice infected with T3SA+ but not T3SA- developed steatorrhea and bilirubinemia. Liver tissue from mice infected with T3SA+ demonstrated intense inflammation focused at intrahepatic bile ducts, pathology analogous to that found in biliary atresia in humans, and high levels of T3SA+ antigen in bile duct epithelial cells. T3SA+ bound 100-fold more efficiently than T3SA- to human cholangiocarcinoma cells. These observations suggest that the carbohydrate-binding specificity of a virus can dramatically alter disease in the host and highlight the need for epidemiologic studies focusing on infection by sialic acid-binding reovirus strains as a possible contributor to the pathogenesis of neonatal biliary atresia.
Figures






References
-
- Tyler, K.L. 2001. Mammalian reoviruses. In Fields virology. 4th edition. D.M. Knipe and P.M. Howley, editors. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 1729–1945.
-
- Virgin, H.W., Tyler, K.L., and Dermody, T.S. 1997. Reovirus. In Viral pathogenesis. N. Nathanson, editor. Lippincott-Raven Publishers. Philadelphia, Pennsylvania, USA. 669–699.
-
- Tardieu M, Weiner HL. Viral receptors on isolated murine and human ependymal cells. Science. 1982;215:419–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA-68485/CA/NCI NIH HHS/United States
- T32 GM-07347/GM/NIGMS NIH HHS/United States
- DK-20593/DK/NIDDK NIH HHS/United States
- R01 AI038296/AI/NIAID NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 AI-38296/AI/NIAID NIH HHS/United States
- T32 AI-07474/AI/NIAID NIH HHS/United States
- T32 AI007474/AI/NIAID NIH HHS/United States
- T32 CA009385/CA/NCI NIH HHS/United States
- T32 CA-09385/CA/NCI NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States

