Probing the catalytic activity of a cell division-specific transpeptidase in vivo with beta-lactams
- PMID: 12813065
- PMCID: PMC161574
- DOI: 10.1128/JB.185.13.3726-3734.2003
Probing the catalytic activity of a cell division-specific transpeptidase in vivo with beta-lactams
Abstract
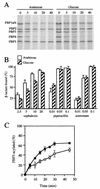
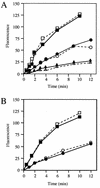
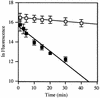
Penicillin-binding protein 3 (PBP3; also called FtsI) is a transpeptidase that catalyzes cross-linking of the peptidoglycan cell wall in the division septum of Escherichia coli. To determine whether the catalytic activity of PBP3 is activated during division, we assayed acylation of PBP3 with three beta-lactams (cephalexin, aztreonam, and piperacillin) in growing cells. Acylation of PBP3 with cephalexin, but not aztreonam or piperacillin, appeared to be stimulated by cell division. Specifically, cephalexin acylated PBP3 about 50% faster in a population of dividing cells than in a population of filamentous cells in which division was inhibited by inactivation or depletion of FtsZ, FtsA, FtsQ, FtsW, or FtsN. However, in a simpler in vitro system using isolated membranes, acylation with cephalexin was not impaired by depletion of FtsW or FtsN. A conflicting previous report that the ftsA3(Ts) allele interferes with acylation of PBP3 was found to be due to the presence of a thermolabile PBP3 in the strain used in that study. The new findings presented here are discussed in light of the hypothesis that the catalytic activity of PBP3 is stimulated by interaction(s) with other division proteins. We suggest that there might be allosteric activation of substrate binding.
Figures


References
-
- Adam, M., C. Fraipont, N. Rhazi, M. Nguyen-Distèche, B. Lakaye, J. M. Frère, B. Devreese, J. Van Beeumen, Y. van Heijenoort, J. van Heijenoort, and J. M. Ghuysen. 1997. The bimodular G57-V577 polypeptide chain of the class B penicillin-binding protein 3 of Escherichia coli catalyzes peptide bond formation from thiol esters and does not catalyze glycan chain polymerization from the lipid II intermediate. J. Bacteriol. 179:6005-6009. - PMC - PubMed
-
- Addinall, S. G., C. Cao, and J. Lutkenhaus. 1997. FtsN, a late recruit to the septum in Escherichia coli. Mol. Microbiol. 25:303-309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

