Membrane interaction of the glycosyltransferase MurG: a special role for cardiolipin
- PMID: 12813070
- PMCID: PMC161595
- DOI: 10.1128/JB.185.13.3773-3779.2003
Membrane interaction of the glycosyltransferase MurG: a special role for cardiolipin
Abstract
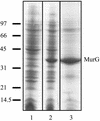
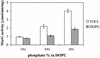
MurG is a peripheral membrane protein that is one of the key enzymes in peptidoglycan biosynthesis. The crystal structure of Escherichia coli MurG (S. Ha, D. Walker, Y. Shi, and S. Walker, Protein Sci. 9:1045-1052, 2000) contains a hydrophobic patch surrounded by basic residues that may represent a membrane association site. To allow investigation of the membrane interaction of MurG on a molecular level, we expressed and purified MurG from E. coli in the absence of detergent. Surprisingly, we found that lipid vesicles copurify with MurG. Freeze fracture electron microscopy of whole cells and lysates suggested that these vesicles are derived from vesicular intracellular membranes that are formed during overexpression. This is the first study which shows that overexpression of a peripheral membrane protein results in formation of additional membranes within the cell. The cardiolipin content of cells overexpressing MurG was increased from 1 +/- 1 to 7 +/- 1 mol% compared to nonoverexpressing cells. The lipids that copurify with MurG were even further enriched in cardiolipin (13 +/- 4 mol%). MurG activity measurements of lipid I, its natural substrate, incorporated in pure lipid vesicles showed that the MurG activity is higher for vesicles containing cardiolipin than for vesicles with phosphatidylglycerol. These findings support the suggestion that MurG interacts with phospholipids of the bacterial membrane. In addition, the results show a special role for cardiolipin in the MurG-membrane interaction.
Figures




References
-
- Arechaga, I., B. Miroux, S. Karrasch, R. Huijbregts, B. de Kruijff, M. J. Runswick, and J. E. Walker. 2000. Characterisation of new intracellular membranes in Escherichia coli accompanying large scale over-production of the b subunit of F1Fo ATP synthase. FEBS Lett. 482:215-219. - PubMed
-
- Armour, G. A., and G. J. Brewer. 1990. Membrane morphogenesis from cloned fragments of bacteriophage PM2 DNA that contain the sp6.6 gene. FASEB J. 4:1488-1493. - PubMed
-
- Barbosa, M. D., H. O. Ross, M. C. Hillman, R. P. Meade, M. G. Kurilla, and D. L. Pompliano. 2002. A multitarget assay for inhibitors of membrane-associated steps of peptidoglycan biosynthesis. Anal. Biochem. 306:17-22. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

