Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle
- PMID: 12813151
- PMCID: PMC2343150
- DOI: 10.1113/jphysiol.2003.041608
Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle
Abstract
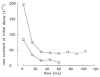
Experiments were carried out to compare the amplitude and time course of Ca2+ release from the sarcoplasmic reticulum (SR) in intact slow-twitch and fast-twitch mouse fibres. Individual fibres within small bundles were injected with furaptra, a low-affinity, rapidly responding Ca2+ indicator. In response to a single action potential at 16 degrees C, the peak amplitude and half-duration of the change in myoplasmic free [Ca2+] (Delta[Ca2+]) differed significantly between fibre types (slow-twitch: peak amplitude, 9.4 +/- 1.0 microM (mean +/- S.E.M.); half-duration, 7.7 +/- 0.6 ms; fast-twitch: peak amplitude 18.5 +/- 0.5 microM; half-duration, 4.9 +/- 0.3 ms). SR Ca2+ release was estimated from Delta[Ca2+] with a computational model that calculated Ca2+ binding to the major myoplasmic Ca2+ buffers (troponin, ATP and parvalbumin); buffer concentrations and reaction rate constants were adjusted to reflect fibre-type differences. In response to an action potential, the total concentration of released Ca2+ (Delta[CaT]) and the peak rate of Ca2+ release ((d/dt)Delta[CaT]) differed about 3-fold between the fibre types (slow-twitch: Delta[CaT], 127 +/- 7 microM; (d/dt)Delta[CaT], 70 +/- 6 microM ms-1; fast-twitch: Delta[CaT], 346 +/- 6 microM; (d/dt)Delta[CaT], 212 +/- 4 microM ms-1). In contrast, the half-duration of (d/dt)Delta[CaT] was very similar in the two fibre types (slow-twitch, 1.8 +/- 0.1 ms; fast-twitch, 1.6 +/- 0.0 ms). When fibres were stimulated with a 5-shock train at 67 Hz, the peaks of (d/dt)Delta[CaT] in response to the second and subsequent shocks were much smaller than that due to the first shock; the later peaks, expressed as a fraction of the amplitude of the first peak, were similar in the two fibre types (slow-twitch, 0.2-0.3; fast-twitch, 0.1-0.3). The results support the conclusion that individual SR Ca2+ release units function similarly in slow-twitch and fast-twitch mammalian fibres.
Figures

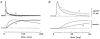
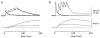


References
-
- Abate F, Bruton JD, De Haan A, Westerblad H. Prolonged force increase following a high-frequency burst is not due to a sustained elevation of [Ca2+]i. Am J Physiol Cell Physiol. 2002;283:C42–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

