Cancer drugs, genetic variation and the glutathione-S-transferase gene family
- PMID: 12814324
- PMCID: PMC9086716
- DOI: 10.2165/00129785-200303030-00002
Cancer drugs, genetic variation and the glutathione-S-transferase gene family
Abstract
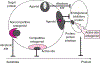
The glutathione-S-transferase (GST) super family comprises multiple isozymes (Alpha, Mu, Pi, Omega, Theta, and Zeta) with compelling evidence of functional polymorphic variation. Over the last two decades, a significant body of data has accumulated linking aberrant expression of GST isozymes with the development and expression of resistance to cancer drugs. Clinical correlation studies show that genetic differences within the human GST isozymes may play a role in cancer susceptibility and treatment. The initial confusion was presented by the fact that not all drugs used to select for resistance were substrates for thioether bond catalysis by GSTs. However, recent evidence that certain GST isozymes possess the capacity to regulate mitogen activated protein kinases presents an alternative explanation. This dual functionality has contributed to the recent efforts to target GSTs with novel small molecule therapeutics. While the ultimate success of these attempts remains to be shown, at least one drug is in late-stage clinical testing. In addition, the concept of designing new drugs that might interfere with protein:protein interactions between GSTs and regulatory kinases provides a novel approach to identify new targets in the search for cancer therapeutics.
Figures
References
-
- Wang AL, Tew KD. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep 1985; 69: 677–82 - PubMed
-
- Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res 1994. Aug 15; 54(16): 4313–20 - PubMed
-
- Tew KD, Monks A, Barone L, et al. Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol Pharmacol 1996. Jul; 50(1): 149–59 - PubMed
-
- Fan M, Chambers TC. Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist Updat 2001. Aug; 4(4): 253–67 - PubMed
-
- Litwack G, Ketterer B, Arias IM. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature 1971; 234: 466–7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
