The small intestine as a model for evaluating adult tissue stem cell drug targets
- PMID: 12814429
- PMCID: PMC6496932
- DOI: 10.1046/j.1365-2184.2003.00264.x
The small intestine as a model for evaluating adult tissue stem cell drug targets
Abstract
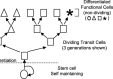
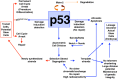
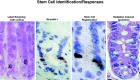
Adult tissue stem cells are defined and some current controversies are discussed. These crucial cells are responsible for all cell production in renewing tissues, and play a vital role in tissue regeneration. Although reliable stem cell markers are generally unavailable for adult epithelial tissues, the small intestinal crypts are an excellent in vivo model system to study stem cells. Within this tissue, the stem cells have a very well-defined cell position, allowing accurate definition of stem cell specific events. Clonal regeneration assays for the small intestine allow stem cell survival and functional competence to be studied. The ultimate lineage ancestor stem cells are extremely efficiently protected from genetic damage, which accounts for the low cancer incidence in this tissue. Some of the regulatory networks governing stem and transit cell behaviour are beginning to be understood and it is postulated that p53 plays a crucial role in these processes.
Figures




References
-
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williams J, Wright NA (2000) Hepatocytes from non‐hepatic adult stem cells. Nature 406, 257. - PubMed
-
- Battle E, Henderson JT, Beghtel H, Born van den MMW, Sancho E, Huls G, Meeldijk J, Robertson J, Wetering van de M, Pawson T, Clevers H (2002) β‐Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/Ephrin B. Cell 111, 251. - PubMed
-
- Bickenbach JR (1981) Identification and behaviour of label retaining cells in oral mucosa and skin. J. Dent. Res. 60, 1611. - PubMed
-
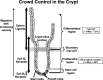
- Booth C, Brady G, Potten CS (2002) Crowd control in the crypt. Nature Med. 8, 1360. - PubMed
-
- Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255, 197. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

