A proof of concept study to evaluate putative benefits of montelukast in moderate persistent asthmatics
- PMID: 12814457
- PMCID: PMC1884256
- DOI: 10.1046/j.1365-2125.2003.01813.x
A proof of concept study to evaluate putative benefits of montelukast in moderate persistent asthmatics
Abstract
Aims: Whether chronic dosing with montelukast confers benefit in patients with moderate to severe asthma remains to be fully established. A proof of concept study was performed evaluating putative benefits with montelukast in moderate persistent asthmatics who were taken off inhaled corticosteroids (ICS) and switched to salmeterol. The latter was done to dissociate the effects of montelukast from ICS.
Methods: Twenty moderate to severe persistent asthmatics completed a randomized double-blind crossover study. Subjects received montelukast 10 mg daily or placebo for 2 weeks each. This was preceded by a 2-week run-in when ICS were discontinued and salmeterol started, and used on a regular basis throughout the study. Measurements were made after run-in and after both randomized treatments.
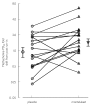
Results: There were no significant sequence effects for responses as to whether placebo or montelukast were given first or second. Methacholine PD20 values after run-in, first and second placebo were 63 micro g, 60 micro g and 64 micro g, respectively (corresponding to 2, 4 and 6 weeks of ICS washout, respectively). Lung function deteriorated pre vs post run-in, which was significant (P < 0.05) for FEF25-75 % predicted. Montelukast conferred significant (P < 0.05) improvements as change from post run-in compared with placebo in methacholine PD20, FEV1 % predicted, FEF25-75 % predicted, diurnal peak expiratory flow, symptoms and salbutamol use. For the primary outcome of methacholine PD20, this amounted to a 1.6-fold difference (95% CI 1.1, 2.5).
Conclusions: In moderate persistent asthmatics switched from taking ICS to salmeterol alone, adding montelukast conferred significant benefits on all parameters of asthma control. Further studies are indicated to evaluate whether montelukast exhibits additive effects to ICS/long-acting beta2-adrenoceptor agonist combination inhalers upon clinically important outcomes.
Figures



References
-
- National Asthma Education and Prevention Program. Expert Panel Report 2Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 1997. Publication no. 97–4051.
-
- The British Guidelines on Asthma Management. 1995 review and position statement. Thorax. 1997;52(1):1–21. - PubMed
-
- Lipworth BJ. Leukotriene-receptor antagonists. Lancet. 1999;353:57–62. - PubMed
-
- Laviolette M, Malmstrom K, Lu S, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Montelukast/Beclomethasone Additivity Group. Am J Respir Crit Care Med. 1999;160:1862–1868. - PubMed
-
- Fish JE, Israel E, Murray JJ, et al. Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest. 2001;120:423–430. - PubMed

